Analysis and detection method of palbociclib intermediates and impurities in palbociclib intermediates
A technology for intermediate and mass analysis, applied in the direction of analyzing materials, measuring devices, material separation, etc., can solve problems such as the reduction of drug solubility, and achieve the effect of effective control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
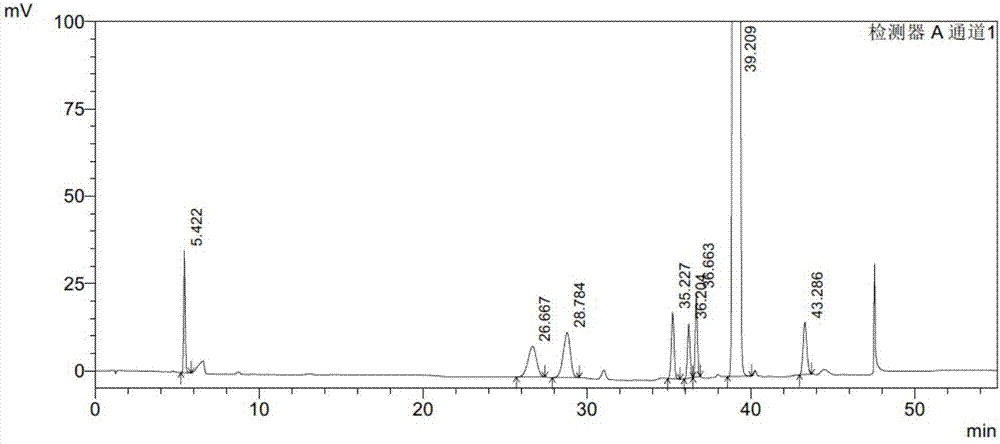
Embodiment 1
[0088] Instruments and conditions:
[0089] Shimadzu LC-2010AHT high performance liquid chromatograph and Shimadzu LC-solution workstation; Octadecylsilane bonded silica gel column (Waters C18, 4.6mm×150mm×5μm); use 0.01M ammonium acetate buffer as mobile phase A (unadjusted pH value is 6.6), acetonitrile as mobile phase B, perform linear gradient elution according to the elution gradient table above The detection wavelength is 235nm; the column temperature of the column oven is 30°C; the flow rate of the mobile phase is 1.0ml / min; the injection volume of the liquid phase analysis is 20μl.
[0090] experiment procedure:
[0091] Preparation of impurity stock solution: take appropriate amount of PB-SM1, PB-SM2, PB-1A, PB-1, PB-2D, PB-2B and PB-2A reference substances, weigh them accurately, add dichloromethane to dissolve and make A solution containing 0.2mg of PB-SM1, 0.2mg of PB-SM2, 0.2mg of PB-1A, 0.2mg of PB-1, 0.2mg of PB-2D, 0.2mg of PB-2B and 0.2mg of PB-2A in each 1...
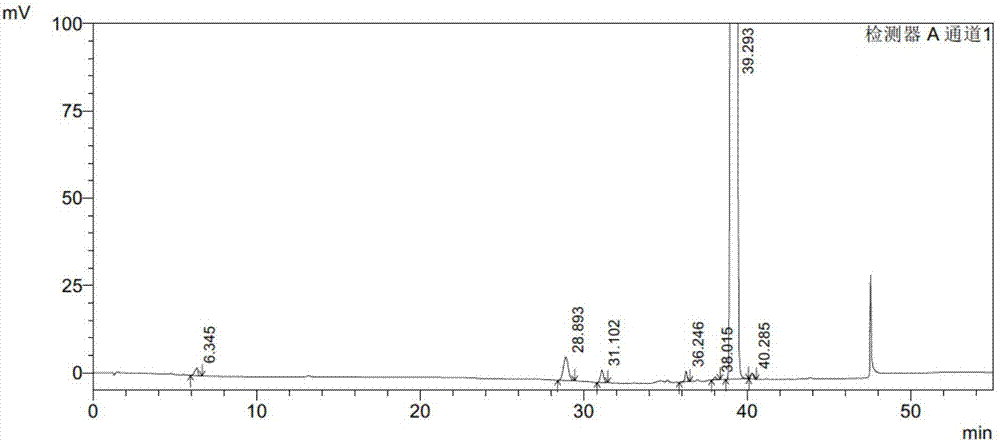
Embodiment 2
[0098] Instruments and conditions:
[0099] Shimadzu LC-2010AHT high performance liquid chromatograph and Shimadzu LC-solution workstation; Octadecylsilane bonded silica gel column (Waters C18, 4.6mm×150mm×5μm); use 0.01M ammonium acetate buffer as mobile phase A, acetonitrile as mobile phase B, perform linear gradient elution according to the above table; detection wavelength is 235nm; column temperature is 30°C ; The flow rate of the mobile phase is 0.9ml / min; the injection volume of the liquid phase analysis is 20μl.
[0100] experiment procedure:
[0101] Preparation of impurity stock solution: take appropriate amount of PB-SM1, PB-SM2, PB-1A, PB-1, PB-2D, PB-2B and PB-2A reference substances, weigh them accurately, add dichloromethane to dissolve and make A solution containing 0.2mg of PB-SM1, 0.2mg of PB-SM2, 0.2mg of PB-1A, 0.2mg of PB-1, 0.2mg of PB-2D, 0.2mg of PB-2B and 0.2mg of PB-2A in each 1ml solution, as Impurity stock solution.
[0102] Preparation of syst...
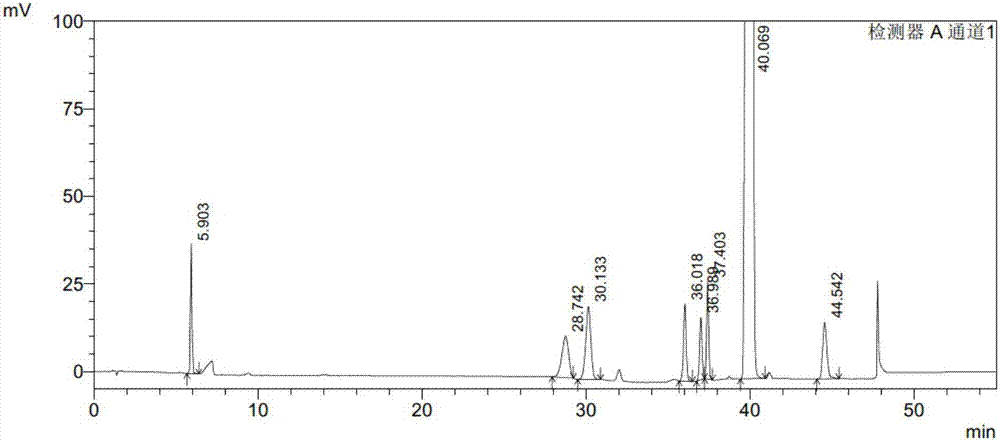
Embodiment 3
[0107] Instruments and conditions:
[0108] Shimadzu LC-2010AHT high performance liquid chromatograph and Shimadzu LC-solution workstation; Octadecylsilane bonded silica gel column (Waters C18, 4.6mm×150mm×5μm); use 0.01M ammonium acetate buffer as mobile phase A, acetonitrile as mobile phase B, perform linear gradient elution according to the above table; detection wavelength is 235nm; column temperature is 30°C ; The flow rate of the mobile phase is 1.1ml / min; the injection volume of the liquid phase analysis is 20μl.
[0109] experiment procedure:
[0110] Preparation of impurity stock solution: take appropriate amount of PB-SM1, PB-SM2, PB-1A, PB-1, PB-2D, PB-2B and PB-2A reference substances, weigh them accurately, add dichloromethane to dissolve and make A solution containing 0.2mg of PB-SM1, 0.2mg of PB-SM2, 0.2mg of PB-1A, 0.2mg of PB-1, 0.2mg of PB-2D, 0.2mg of PB-2B and 0.2mg of PB-2A in each 1ml solution, as Impurity stock solution.
[0111] Preparation of syst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com