Schiff base fluorescent probe and synthesis method and application thereof
A technique for fluorescent probes and synthesis methods, applied in the field of Schiff base fluorescent probes and their synthesis and application, capable of solving problems such as limited application range, long response time, and poor selectivity, achieving clear and recognizable results and low detection costs , Simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis of embodiment 1 Schiff base fluorescent probe L
[0033] 4-phenyl-3-thiosemicarbazide (0.167g, 1mmol) was dissolved in ethanol (5ml); take 8-hydroxyjulonidine-9-formaldehyde (0.217g, 1mmol) in a three-necked flask, add 5ml EtOH, heating to reflux, the solution turns into a yellow clear solution at 50°C; at this time, add the ethanol solution of 4-phenyl-3-thiosemicarbazide to the ethanol solution of 8-hydroxyjulonidine-9-formaldehyde, and keep the temperature The reaction was continued at 80° C. After 24 hours, a large amount of yellow solid was produced, which was filtered by suction and dried to obtain the Schiff base fluorescent probe L (0.242 g, yield: 66%). 1 HNMR(DMSO-d6)δ:11.385(s,1H,-OH),9.903(s,1H,-NH-),9.297(s,1H,-NH-),8.156(s,1H,-HC=N ),7.498(d,2H,-ArH),7.339(t,2H,-ArH),7.166(t,1H,-ArH),3.159(m,4H,-CH 2 ),2.593(m,4H,-CH 2 ),1.852(m,4H,-CH 2 ), HRMS (ESI): C 20 h 22 N 4 OS(M+H) calculated value 367.1514, experimental value 367.1587.
Embodiment 2
[0034] Embodiment 2 Schiff base fluorescent probe L is used for the fluorescence spectrometry of trace water
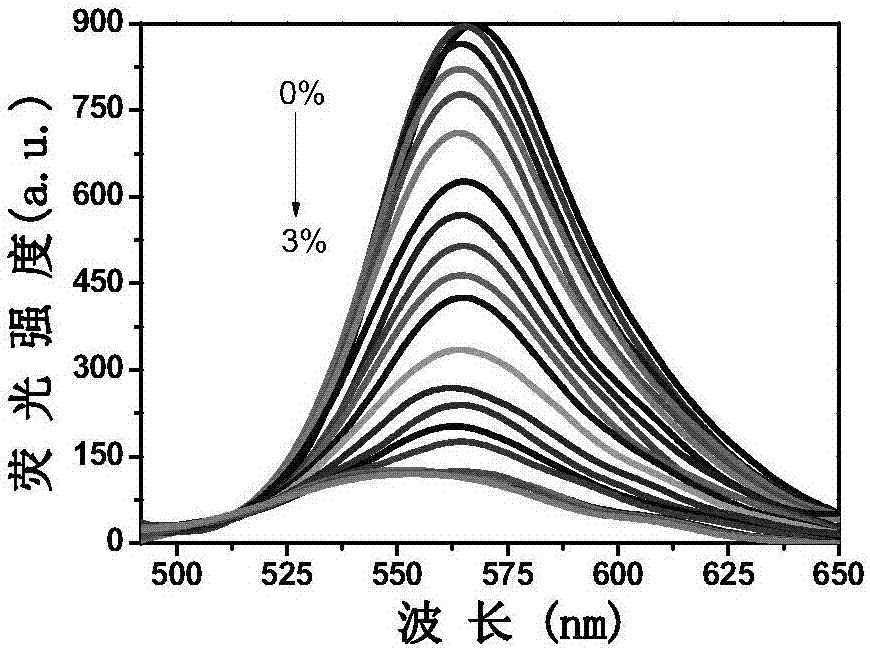
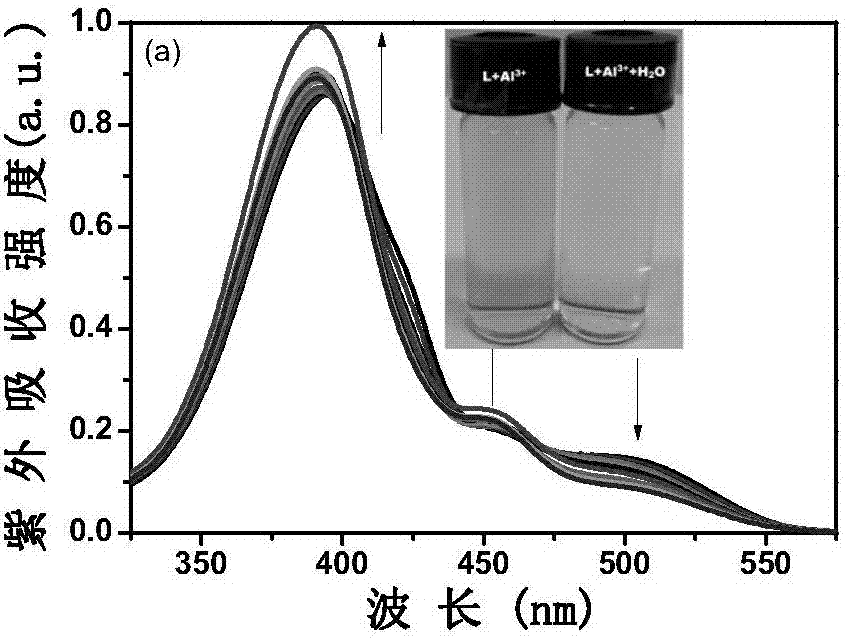
[0035] Take 20 μL of probe L stock solution and add it to a clean colorimetric tube, then add 12 μL of Al 3+ Finally, dilute to 5mL with methanol, shake the solution, take 2.5mL into a clean cuvette, and detect it on a fluorescence spectrophotometer. With the addition of a small amount of water, the solution gradually changes from orange to yellow. It is yellow, and the emission peak at 570nm gradually weakens, and a blue shift occurs, moving from 570nm to 540nm. For the fluorescence spectrum, see figure 1 .
Embodiment 3
[0036] Embodiment 3 Schiff base fluorescent probe L measures the linear relationship of trace water
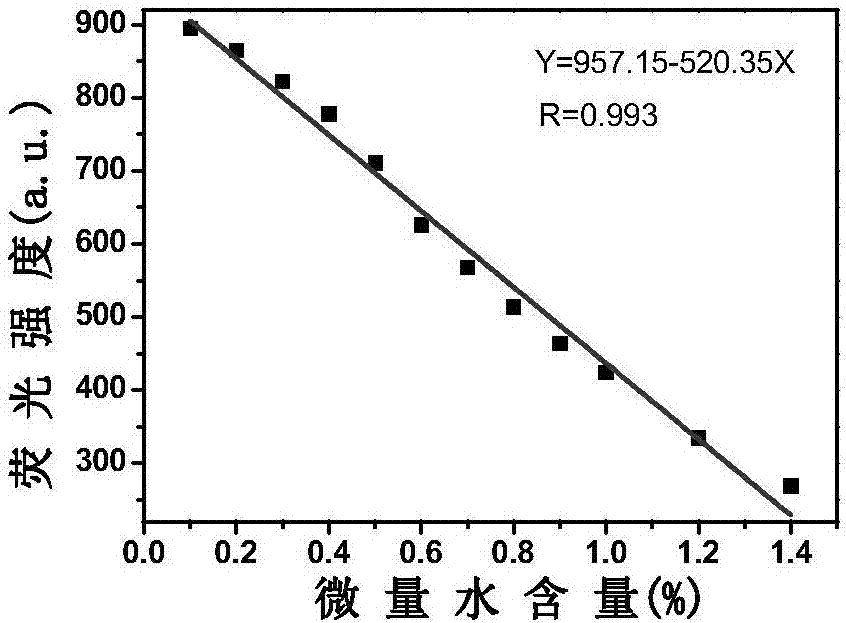
[0037] Take 20 μL of probe L stock solution and add it to a clean colorimetric tube, then add 12 μL of Al 3+ Methanol solution, finally dilute to 5mL with methanol, shake the solution evenly, take 2.5mL into a clean cuvette, and detect it on a fluorescence spectrophotometer. With the addition of a small amount of water, the solution gradually changes from orange to yellow. Yellow, and the emission peak at 570nm gradually weakens, and a blue shift occurs, moving from 570nm to 540nm. The amount of gradually adding trace water is 0%-3%, and the fluorescence intensity F of the system and the content of trace water present a good linear relationship in the scope of 0.1%-1.4% (R 2 = 0.986). Take the trace water content as the abscissa, and take the fluorescence intensity F as the ordinate to plot, and obtain a linear equation: F=957.15-520.35 [H 2 O]. See the working line diagra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com