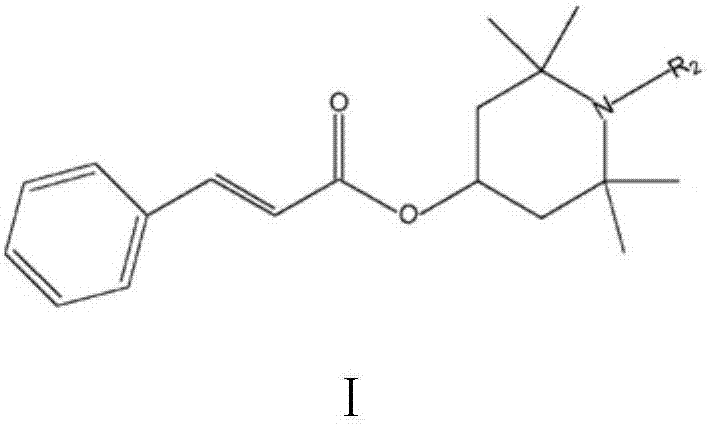
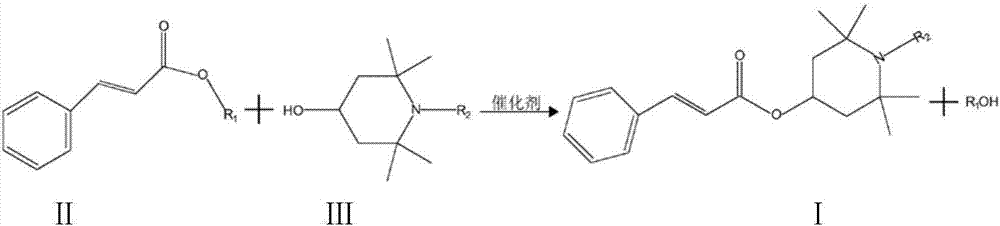
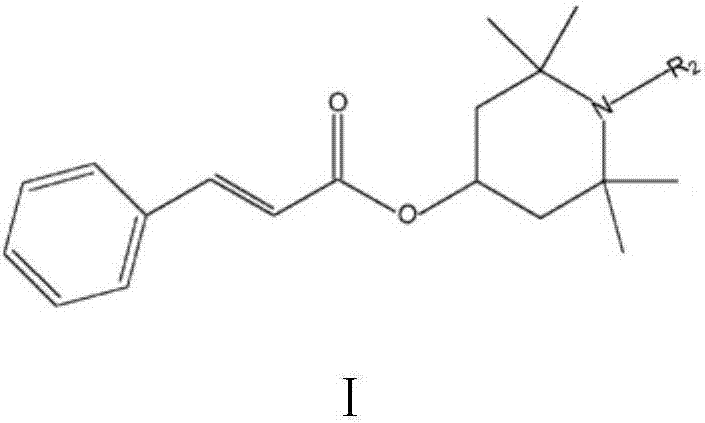
Reactive type light stabilizer and preparation method thereof
A light stabilizer and reactive technology, applied in the field of reactive light stabilizers and their preparation, can solve the problems of high toxicity, easy self-polymerization, etc., to avoid self-polymerization, good light stabilization effect, and small steric hindrance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Reactants: ethyl cinnamate and tetramethylpiperidinol;
[0028] Catalyst: sodium methoxide;
[0029] Organic solvent: n-heptane;
[0030] The substance molar ratio of ethyl cinnamate and tetramethylpiperidinol is 1:1;
[0031] The mass ratio of catalyst and ethyl cinnamate is 0.003:1;
[0032] The mass ratio of organic solvent and ethyl cinnamate is 1:1;
[0033] Put the reactant and organic solvent into the reaction kettle and reflux water separation at 100°C; after the reflux water separation is completed, put in the catalyst and heat the reaction. End (GC monitoring reaction);
[0034] Wash, separate, decolorize, and filter the reacted material; then distill off the organic solvent under reduced pressure to obtain 275 g of hindered amine light stabilizer with a content (GC) of 99.5% and a yield of 95.8%.
Embodiment 2
[0036] Reactants: propyl cinnamate and pentamethylpiperidinol;
[0037] Catalyst: aluminum ethylate;
[0038] Organic solvent: toluene;
[0039] The substance ratio of propyl cinnamate and pentamethylpiperidinol is 1:1.5;
[0040] The mass ratio of catalyst and propyl cinnamate is 0.5:1;
[0041] The mass ratio of organic solvent and propyl cinnamate is 10:1;
[0042] Put the reactant and organic solvent into the reaction kettle and reflux water separation at 100°C; after the reflux water separation is completed, put in the catalyst, heat the reaction, the reaction temperature is 140°C, continue to flow out to bring out the propanol, and at the same time properly replenish the solvent until Reaction ends (GC monitors reaction);
[0043] Wash, separate, decolorize, and filter the reacted material; then distill off the organic solvent under reduced pressure to obtain 286 g of hindered amine light stabilizer with a content (GC) of 99.2% and a yield of 95%.
Embodiment 3
[0045] Reactants: methyl cinnamate and tetramethylpiperidinol;
[0046] Catalyst: tetrabutyl titanate;
[0047] Organic solvent: petroleum ether (boiling range: 90-120°C);
[0048] The molar ratio of methyl cinnamate and tetramethylpiperidinol is 1:1.05;
[0049] The mass ratio of catalyst and methyl cinnamate is 0.01:1;
[0050] The mass ratio of organic solvent to methyl cinnamate is 1:1;
[0051] Put the reactant and organic solvent into the reaction kettle and reflux water separation at 100°C; after the reflux water separation is completed, put in the catalyst, heat the reaction, the reaction temperature is 130°C, continue to reflux to separate methanol until the end of the reaction (GC monitors the reaction) ;
[0052] Wash, separate, decolorize, and filter the reacted material; then distill off the organic solvent under reduced pressure to obtain 273 g of hindered amine light stabilizer with a content (GC) of 99.1% and a yield of 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com