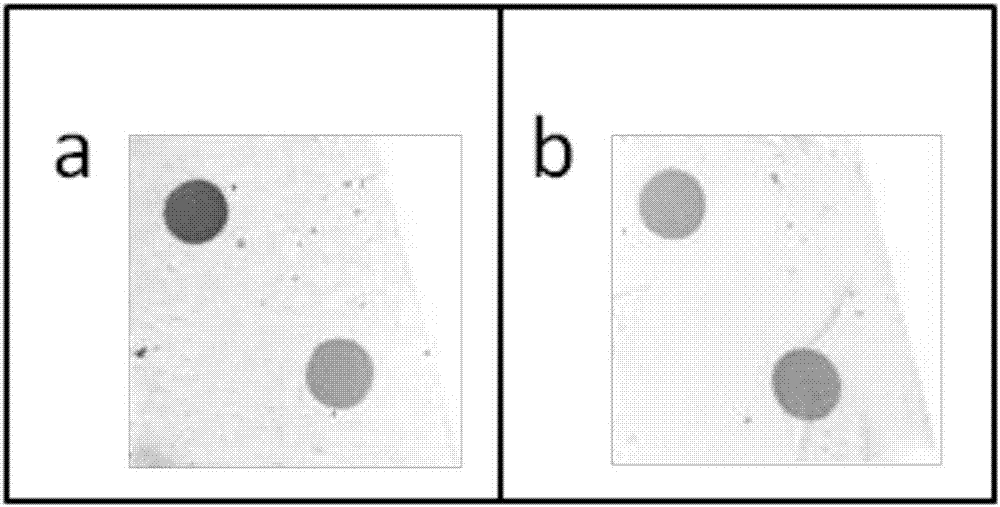
Human body anti-AQP4 autoantibodies detection material and preparation method thereof
A technology for detecting materials and autoantibodies, applied in the field of biomedicine, can solve the problems of low sensitivity and specificity of the CBA method, difficult to clearly interpret the detection results, and complicated operation process, so as to increase the detection sensitivity and specificity, and interpret the detection results. Easy to identify, reduce the effect of non-specific signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be further described in detail below in conjunction with specific embodiments.
[0032] The preparation method of the human anti-AQP4 autoantibody detection material provided by the present invention specifically comprises the following steps:
[0033] 1. Artificially synthesized or obtained AQP4 full-length target gene by PCR method, construct HEK293 / pCI-neo-M1-AQP4, HEK293 / pCI-neo-M23-AQP4 cells stably or transiently expressing M1 and M23 subtypes, and empty them HEK293 / pCI-neo cells were used as the control group. The specific steps are as follows:
[0034] 1. Plasmid construction
[0035] 1.1 Obtaining the target gene: obtain the full-length target gene of AQP4 of M1 and M23 subtypes by artificial synthesis or PCR method, and add NotI / NheI enzyme cutting sites at both ends.
[0036] 1.2 Insert the obtained two target genes into the vector pCI-neo plasmid respectively, the insertion site is NotI / NheI, name the obtained two plasmids respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com