Preparing method of bazedoxifene derivative
A technology for bazedoxifene and derivatives, which is applied in the field of compound preparation and achieves the effects of simple operation method, important application value and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
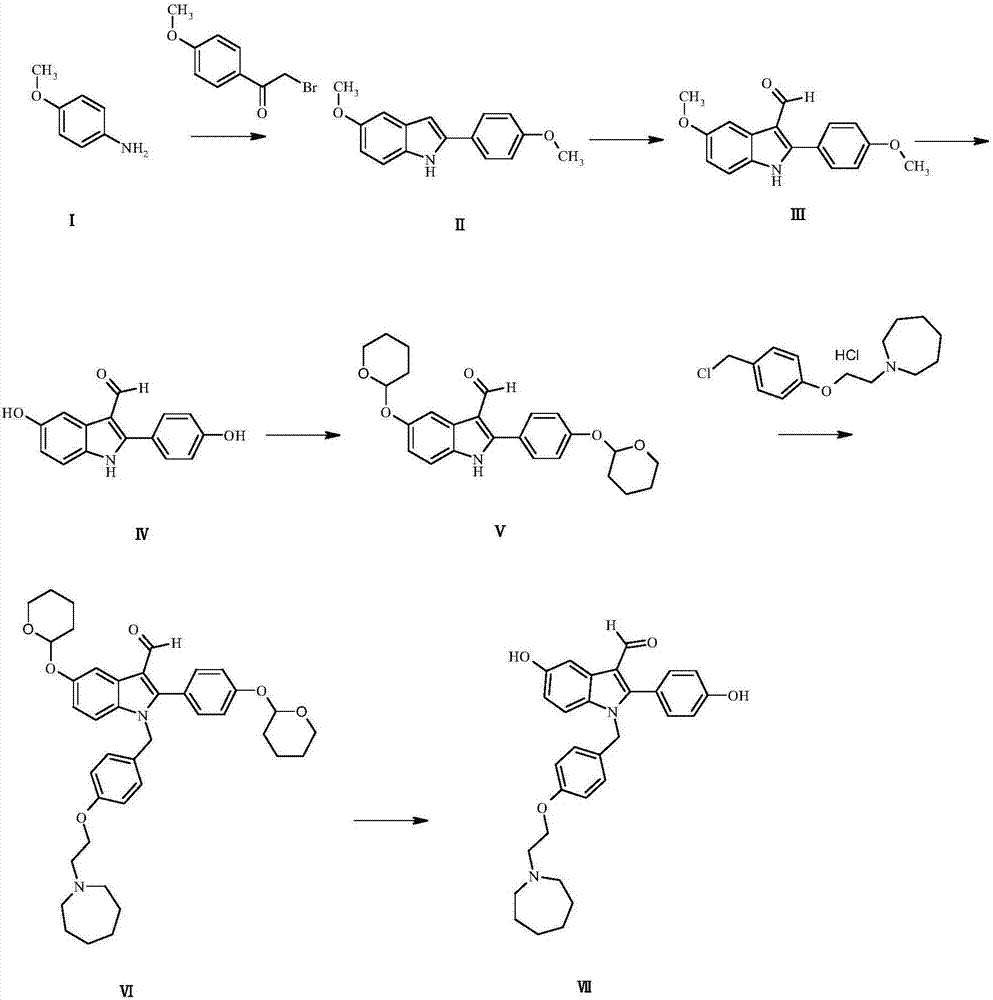
[0060] Such as figure 1 Shown: the preparation method of bazedoxifene derivative, specifically comprises the following steps:
[0061] Preparation of Compound II:
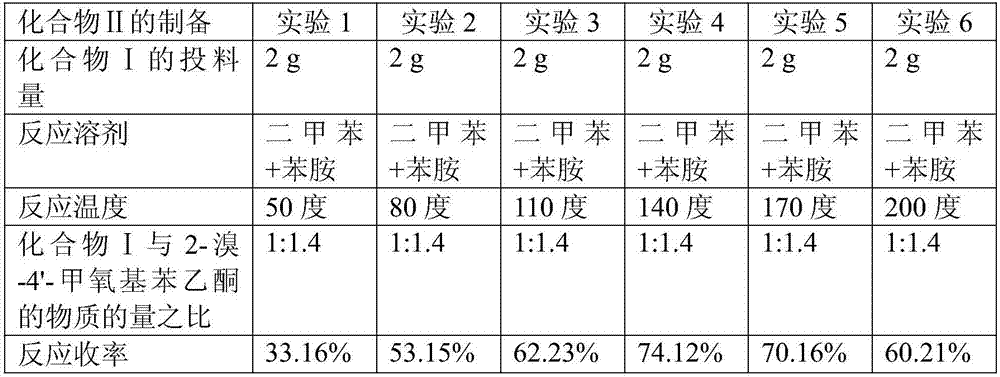
[0062] Dissolve p-methoxyaniline I (46.50g, 0.0.38mol) in aniline (25mL), add 2-bromo-4'-methoxyacetophenone (61.78g, 0.27mol) in xylene (93mL) solution, reacted at 140°C for 9 hours, and the light reddish brown solution turned into a deep reddish brown solution. Under ice-cooling, 2N HCl aqueous solution (400 mL) was added, extracted with ethyl acetate (200 mLx2), the organic phase was dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 51.65 g of light reddish-brown liquid Compound II with a yield of 75.34%.
[0063]
[0064] Preparation of Compound III: Dissolve Compound II (49.21g, 0.19mol) in N,N-dimethylformamide (900mL), add phosphorus oxychloride (44.68g, 0.29mol) dropwise, and react at 65°C for 7 hours , the light reddish brown solution turned into dark yellow clear solution. Un...
Embodiment 2
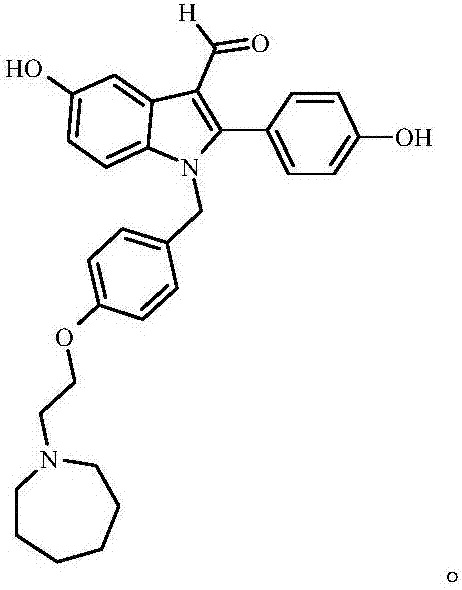
[0075] Preliminary experiments were carried out on Compound VII--Bazedoxifene derivatives prepared in Example 1 of the present invention. The experimental results showed that Compound VII synthesized by the present invention can competitively inhibit the binding of 17β-estradiol to ERα and ERβ. It is expected to be developed as a drug for treating postmenopausal women with moderate to severe hot flashes and preventing osteoporosis, or for studying the pharmacokinetics and metabolic pathways of bazedoxifene-related drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com