Colorimetric fluorescent probe for supersensitive analysis of mercury ions and preparation method thereof
A technology of mercury ions and preparations, which is applied in the fields of analyzing materials, fluorescence/phosphorescence, and material excitation analysis, etc. It can solve the problems of complex synthesis, insufficient selectivity, and insufficient response speed, etc., and achieve simple synthesis, good stability, and effective The effect of promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] (Scheme 1) Dissolve 387mg (1mmol) of Rhodamine B hybridized fluorescein in 15mL of dichloromethane, then add 337mg (2mmol) of phenyl thiochloroformate and stir for 6h at room temperature, then use a rotary evaporator to perform rotary evaporation. A crude product is obtained. If a purer product is to be obtained, it can be recrystallized in a mixed system of dichloromethane and petroleum ether (eg v / v, 1:5) to obtain a pure product. Obtained 364 mg of red pure product with a yield of 64%.
[0035] (Scheme 2) Dissolve 387mg (1mmol) of rhodamine B hybridized fluorescein in 15mL of dichloromethane, then add 252.7mg (1.5mmol) of phenyl thiochloroformate and stir for 6h at room temperature, then use a rotary evaporator to spin Steamed to obtain crude product. If a purer product is to be obtained, it can be recrystallized in a mixed system of dichloromethane and petroleum ether (eg v / v, 1:5) to obtain a pure product. Obtained 297 mg of red pure product with a y...
Embodiment 2
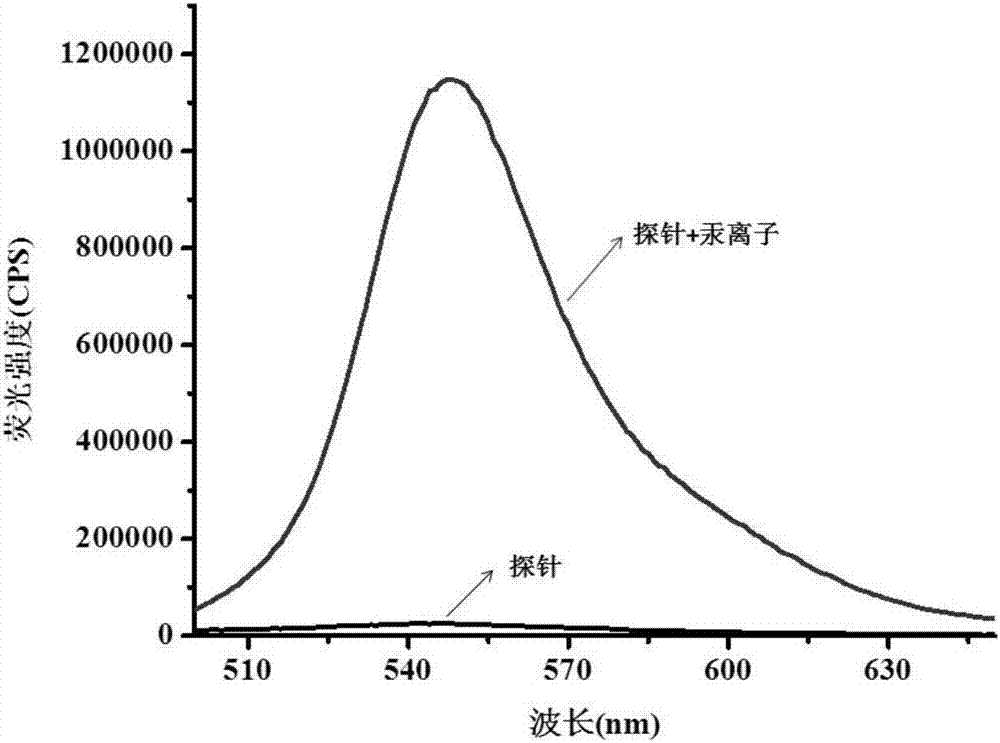
[0041] figure 1 is the probe (5μM) added with Hg 2+ Fluorescence spectra before and after (10 μM). It can be clearly seen from the figure that the addition of mercury ions leads to enhanced fluorescence intensity.
Embodiment 3
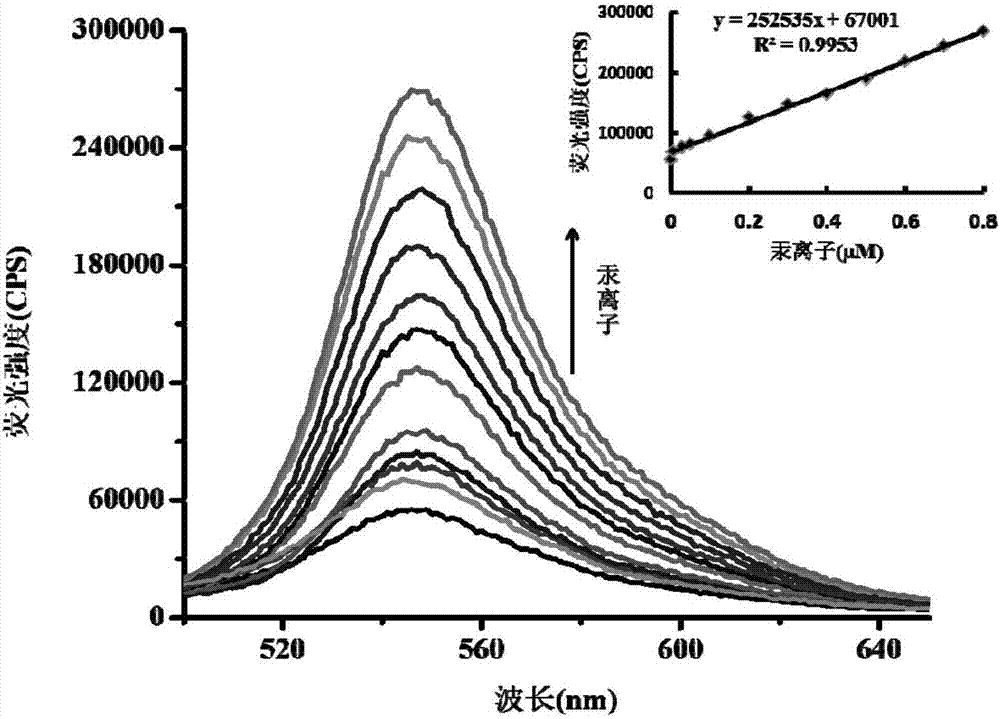
[0043] Figure 2a are different concentrations of Hg 2+ (0-0.8μM) on the probe (5μM) fluorescence spectrum; Figure 2b are different concentrations of Hg 2 + (0-10μM) on the probe (10μM) UV absorption spectrum.
[0044] Depend on Figure 2a It can be seen that with the Hg in the probe solution 2+ As the concentration increases, the fluorescence intensity gradually increases, and at (0-0.8μM)Hg 2+ Concentration range, Hg 2+ There is a good linear relationship between the concentration and the fluorescence intensity. This proves that with the aid of this fluorescent probe the Hg 2+ Quantitative analysis with high sensitivity. Hg in drinking water standard in my country 2+ The standard limit of Hg is 0.001mg / L. Therefore, the probe of the present invention can more accurately determine the Hg in the sample to be tested. 2+ content. Furthermore, with Hg 2+ As the concentration increases, the absorption spectrum gradually increases, which indicates that the colorimetri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com