Peptide reagents and methods for detection and targeting of dysplasia, early cancer and cancer
A reagent and cancer technology, applied in chemical instruments and methods, preparations for in vivo experiments, peptides, etc., can solve problems such as immunogenicity of antibodies and antibody fragments, production cost half-life limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Expression of EGFR extracellular domain (ECD)
[0105] The extracellular domain (ECD) of EGFR (amino acids 1-645 in domains 1-4) was cloned into the pDual GC mammalian expression vector (Stratagene, #214503). The gene was inserted between two Eam1104I restriction sites, creating a unidirectional junction. The CMV promoter drives protein expression in mammalian cells. Myc and His tags were expressed in frame on the C-terminus of recombinant EGFR-ECD for protein characterization and purification, respectively. The thrombin recognition site between EGFR-ECD and myc-His allows the tag to be cleaved after use. Correct construction was verified by DNA sequencing. The constructs were first transiently transfected into HEK-293T cells and verified on Western blot. The constructs were then introduced into Chinese Hamster Ovary (CHO) cells grown in MEMα. Stable clones were established by geneticin selection, and those with the highest expression levels were propagated and cul...
Embodiment 2
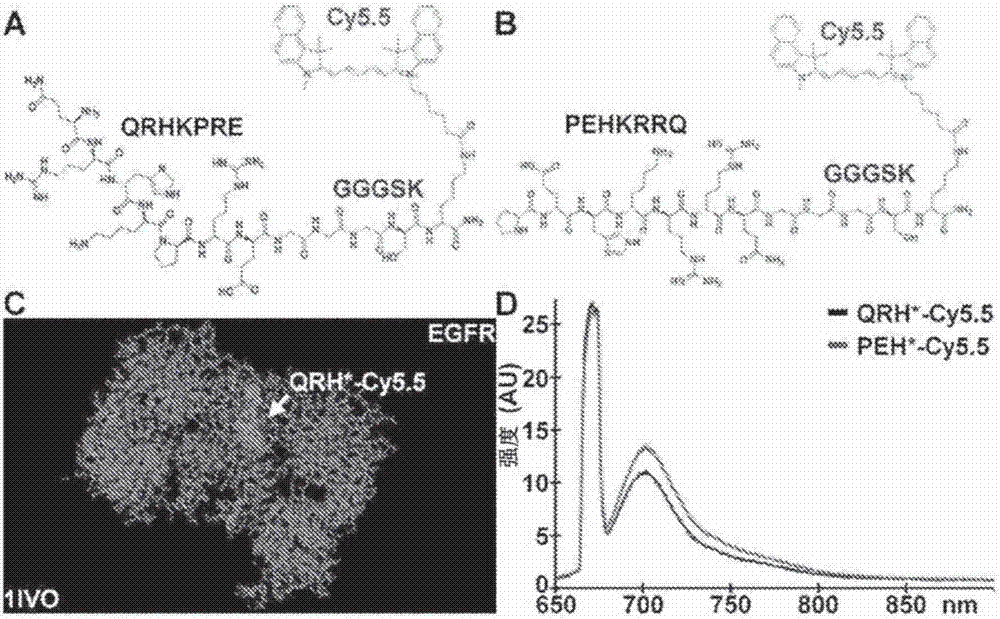
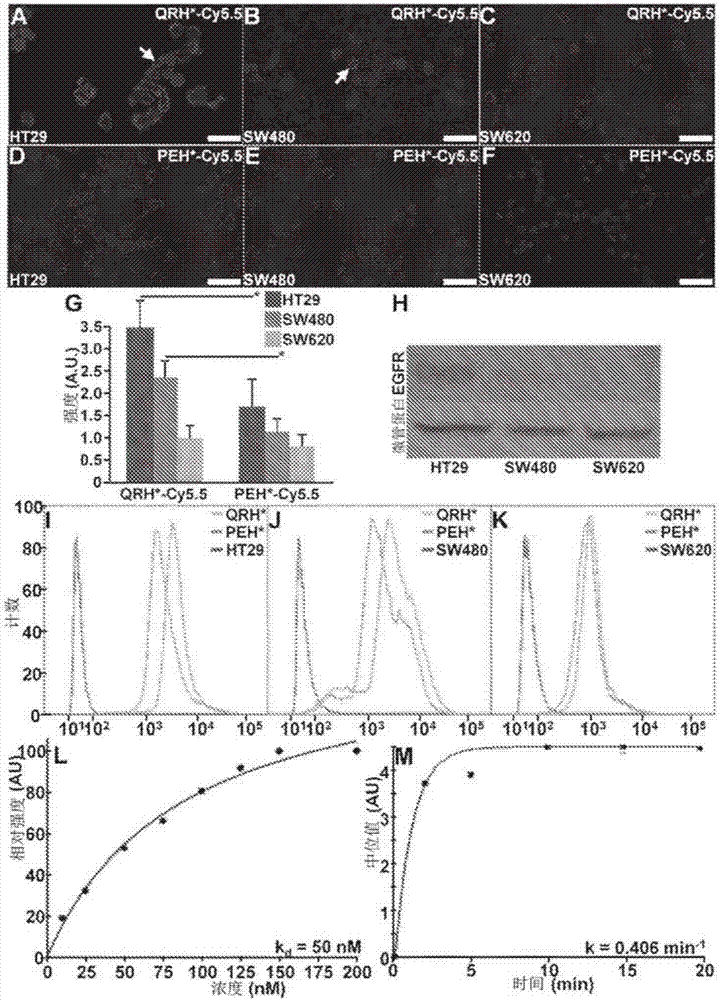
[0109] Peptides specific for EGFR
[0110] Use by express about 10 9 A phage display library (New England Biolabs, Ph.D.-12) consisting of M13 bacteriophages of unique 12-amino acid sequence was used, and peptide selection was performed according to the manufacturer's instructions. Phage library (composed of 2 × 10 9 2×10 unique clones 11 pfu, with about 100 copies per unique clone) for biopanning. Unlike the unbiased protocol performed in Li et al., Gastroenterology, 139:1472-80 (2010), biopanning was performed with a known protein target (EGFR-ECD). Four rounds of biopanning were performed using decreasing amounts (100, 80, 60 and 40 μg) of EGFR-ECD in successive rounds to increase binding specificity. After four rounds of biopanning, 50 phage colonies were randomly selected for DNA preparation and sequencing analysis. These sequences were analyzed with an ABI automatic DNA analyzer (Applied Biosystems) using a primer 5'-CCCTCATAG TTA GCG TAA CG-3' (-96gIII sequencing p...
Embodiment 3
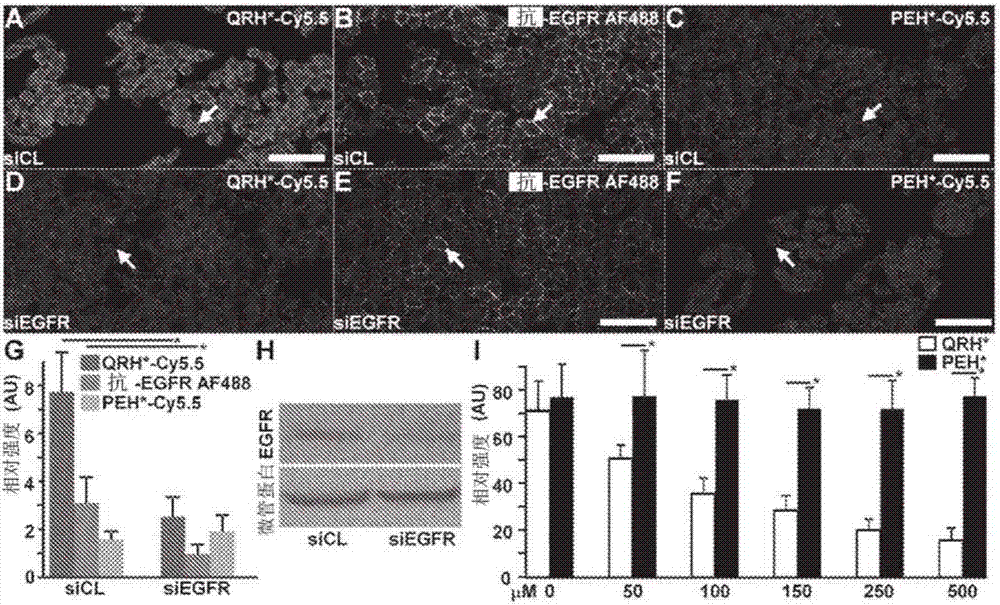
[0116] siRNA knockdown of EGFR
[0117]We knocked down EGFR expression in HT29 cells using siRNA. We used ON-TARGETplus human EGFR siRNA and ON-TARGETplus non-targeting library (Thermo Scientific) following the manufacturer's protocol. 5 μL of siRNA (at a concentration of 5 μM / L) was transfected into HT29 cells using DharmaFECT (Thermo Scientific). QRH*-Cy5.5 and PEH*-Cy5.5 were incubated with HT29siEGFR and siCL cells. Cells were fixed with 4% PFA or methanol. A 1:1000 dilution of the primary monoclonal mouse anti-EGFR antibody (Thermo Scientific, #MS-396, clone 199.12, IgG2a isotype) was incubated overnight at 4°C. Thereafter, cells were washed 3 times with PBS and further incubated with a 1:500 dilution of AF488-labeled secondary goat anti-mouse IgG antibody (Life Technologies, #A-11029) for 1 hour at room temperature, washed 3 times and then fixed on slides with ProLong Gold reagent containing DAPI (Invitrogen). Confocal fluorescence images were collected with FITC, C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com