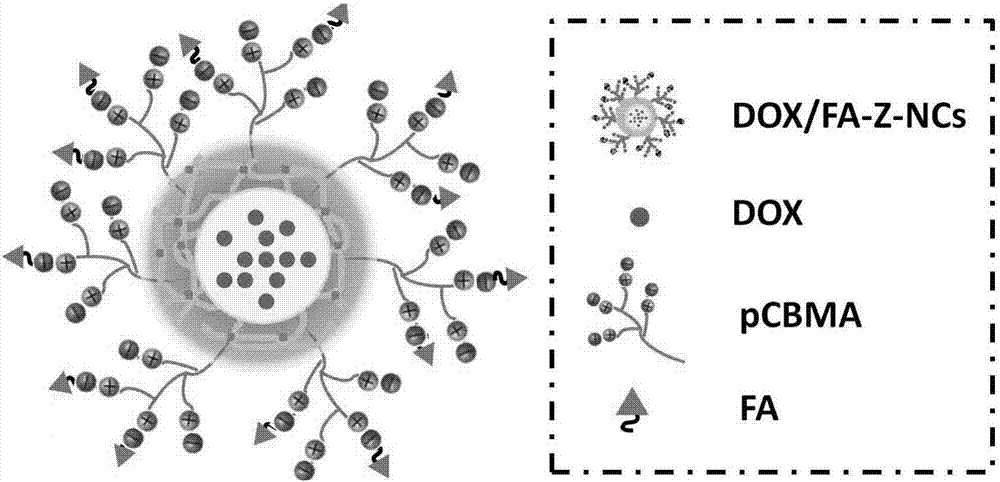
Microenvironment-sensitive drug-loading nano capsules with tumor cell biological reducibility and preparation method thereof
A drug-loaded nano- and tumor cell technology, applied in the field of biomedical materials, can solve the problems of normal tissue side effects, low safety, and poor biocompatibility of loaded nanocapsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
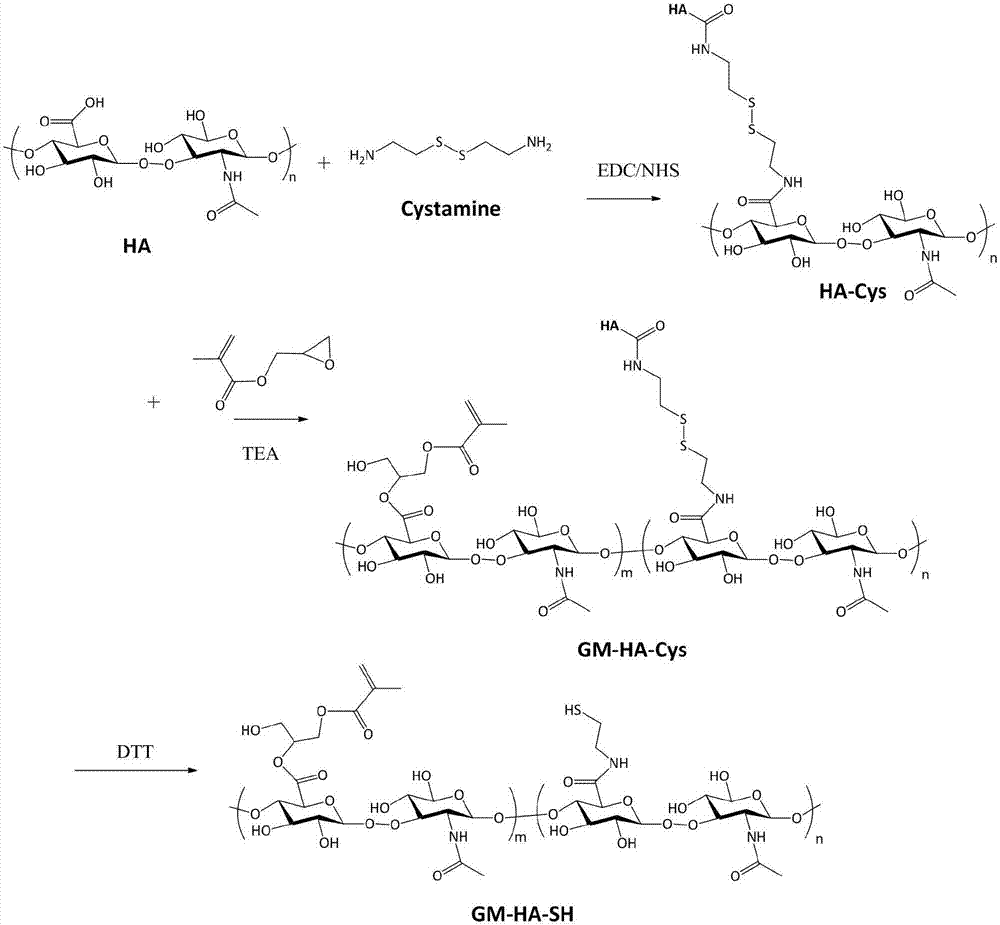
[0051] The preparation of the hyaluronic acid (HA-Cys) that embodiment 1 cystamine binds
[0052] Dissolve 5g of hyaluronic acid in 250ml of PBS (0.01mol / L, pH=7.4), add 3 equivalents of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and 3 equivalents 1-Hydroxybenzotriazole, stirred at room temperature, activated carboxyl group for 4 hours; then added 3 times equivalent of cystamine dihydrochloride, stirred and reacted at room temperature for 24 hours, and then dialyzed with deionized water for 72 hours (the molecular weight cut-off of the dialysis bag was 3500 channels Dayton), to remove unreacted molecules and condensing agents, etc.; among them, hyaluronic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide, 1-hydroxybenzotriazole and cystamine The molar ratio of dihydrochloride is 1:3:3:3.
Embodiment 2
[0053] Example 2 Preparation of hyaluronic acid (GM-HA-SH) combined with esterified cystamine
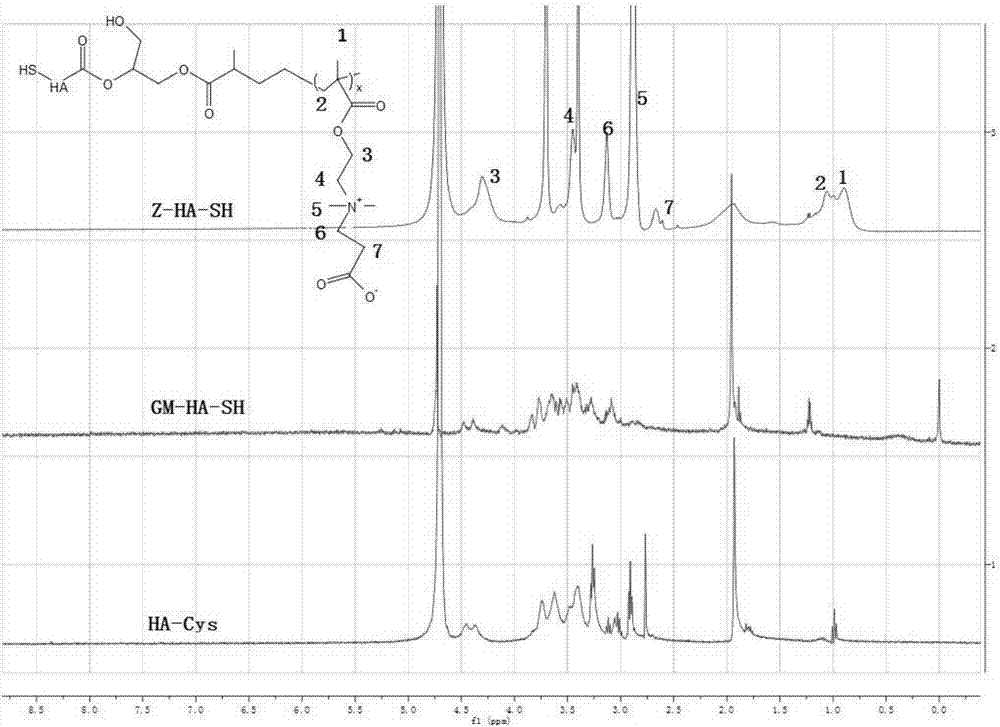
[0054] Dissolve 1 g of cystamine-conjugated hyaluronic acid (HA-Cys) in 25 ml of PBS / dimethylformamide mixture (the volume ratio of PBS and dimethylformamide is 4:1), and add excess methacrylic acid Glycidyl ester and triethylamine were stirred and reacted at room temperature for 14 days. After the reaction was over, dialyzed with deionized water for 72 hours (molecular weight cut-off of the dialysis bag was 3500 Daltons), and then excessive dithiothreitol was added, and the reaction was carried out at room temperature. Stir for 10 hours, then dialysis and purify with deionized water for 72 hours (the molecular weight cut-off of the dialysis bag is 3500 Daltons), and finally freeze-dry to obtain the hyaluronic acid (GM-HA-SH ); wherein the molar ratio of cystamine-bound hyaluronic acid, glycidyl methacrylate, triethylamine and dithiothreitol is 1:20:20:5. The reaction process diagr...
Embodiment 3
[0055] Example 3 Preparation of Multifunctional Hyaluronic Acid (Z-HA-SH)
[0056] Preparation of carboxybetaine methacrylate monomer by ring-opening reaction of 2-(dimethylamino)ethyl methacrylate and β-propiolactone. The specific method is:
[0057] ①Dissolve 30mmol of 2-(dimethylamino)ethyl methacrylate in 150ml of anhydrous acetone;
[0058] ②Under nitrogen protection conditions, dissolve 36mmol of β-propiolactone in 30ml of anhydrous acetone;
[0059] ③ Add the solution obtained in step ② dropwise to the solution obtained in step ①, keep the dropping rate at 1 drop / second, control the dropping time within 15 minutes, then stir and react for 5 hours, and use an ice bath to reduce the temperature of the reaction system during the entire reaction process. Keep at 10°C;
[0060] ④ After the reaction, use anhydrous acetone to precipitate the product, and use anhydrous acetone and anhydrous ether to wash alternately for several times, and then dry it in a vacuum oven to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com