Ultra-sensitive high-selectivity hypochlorous acid colorimetric fluorescence probe
A compound and straight-chain technology, applied in fluorescence/phosphorescence, luminescent materials, and material analysis through optical means, can solve the problems of complex synthesis, poor selectivity, and low sensitivity, and achieve simple synthesis, good stability, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] (Scheme 1) Dissolve 1.6017g (10mmol) 1,6-dihydroxynaphthalene and 3.1335g (10mmol) 4-diethylamino ketoacid in (10mL) trifluoroacetic acid (TFA), then heat to reflux for 24h, and then Add 15 mL of ethyl acetate and heat to reflux for 15 min, cool to room temperature, recrystallize and filter with suction to obtain 3.9420 g of pure product with a yield of 90%.
[0040] (Scheme 2) Dissolve 1.6017g (10mmol) 1,6-dihydroxynaphthalene and 3.4469g (11 mmol) 4-diethylamino ketoacid in (10mL) trifluoroacetic acid (TFA), then heat to reflux for 24h, and then Add 15 mL of ethyl acetate and heat to reflux for 15 min, cool to room temperature, recrystallize and filter with suction to obtain 3.9858 g of pure product with a yield of 91%.
[0041] (Scheme 3) Dissolve 1.6017g (10mmol) 1,6-dihydroxynaphthalene and 3.7602g (12 mmol) 4-diethylamino ketoacid in (10mL) trifluoroacetic acid (TFA), then heat to reflux for 24h, and then Add 15 mL of ethyl acetate and heat to reflux fo...
Embodiment 2
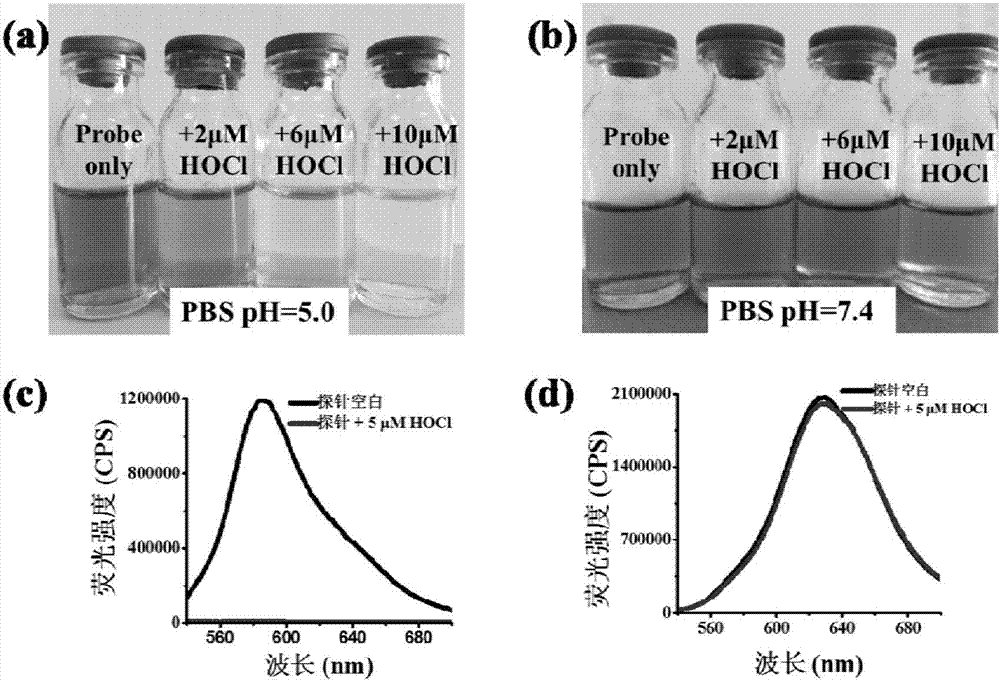
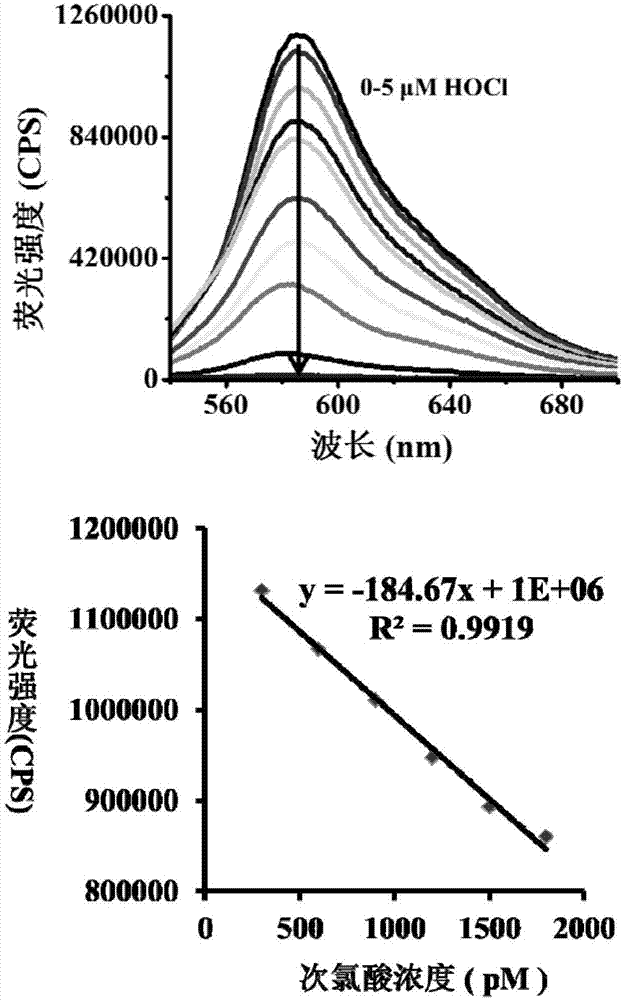
[0046] The inventor of the present invention has carried out following test: (a) under the condition of PBS pH=5.0 hypochlorous acid of different concentrations is to the influence of probe (20 μ M) solution color; (b) under the condition of PBS pH=7.4 hypochlorous of different concentrations The effect of acid on the color of the probe (20μM) solution; (c) is the effect of HOCl (5μM) on the fluorescence spectrum of the probe (5μM) under the condition of PBS pH=5.0; (d) is the effect of HOCl under the condition of PBS pH=7.4 (5 μM) on the fluorescence spectrum of the probe (5 μM). The above measurements were carried out in a pure aqueous solution of 10 mM PBS, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25° C. after the addition of HOCl for 1 min. See Figure 1 for the results.
[0047] From figure 1 (a) as can be seen, the hypochlorous acid that concentration increases gradually under the condition of PBS pH=5.0 can make probe ...
Embodiment 3
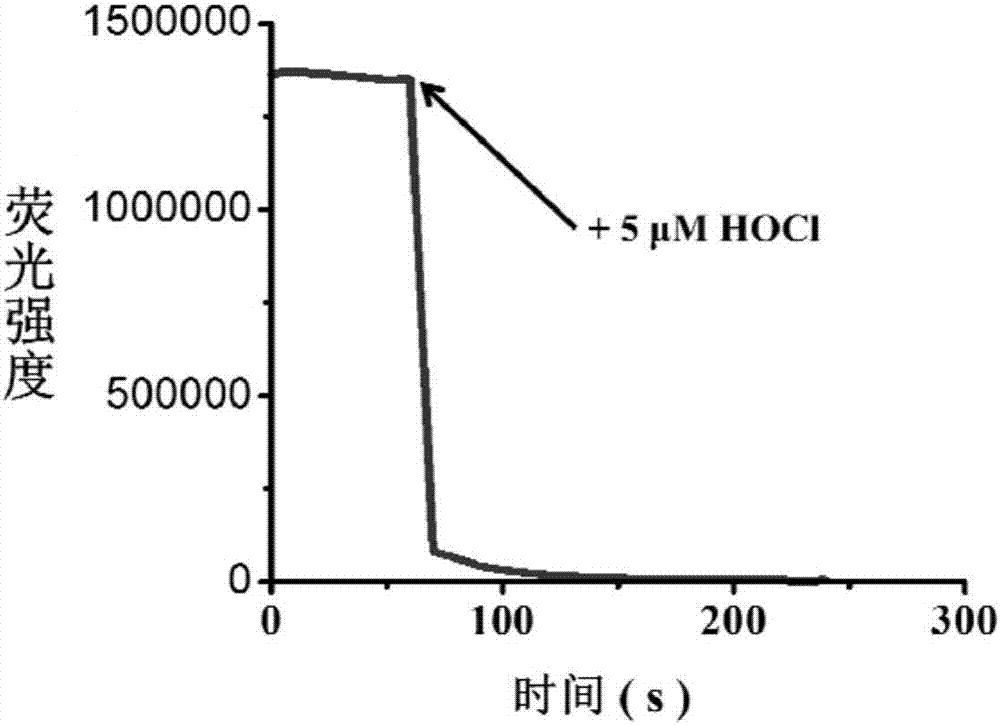
[0049] Test results of probe (5 μM) response time to HOCl (5 μM). The above-mentioned determination is carried out in 10mM PBS, the aqueous solution of pH=5.0, and the probe used is the probe prepared in embodiment 1, and all spectral tests are all measured under 25 ℃. See results figure 2 .
[0050] From figure 2 It can be seen that after adding isochlorous acid, the fluorescence intensity drops suddenly, and the response can be completed within 5s.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com