Monoclonal antibody against muramyl peptides in prevention and treatment of immune-mediated diseases
A therapeutic and preventive technology, applied in the field of immunology and immune-mediated diseases, which can solve the problem of lack of response to therapeutic biologics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 - Characterization of mAbs against MDP
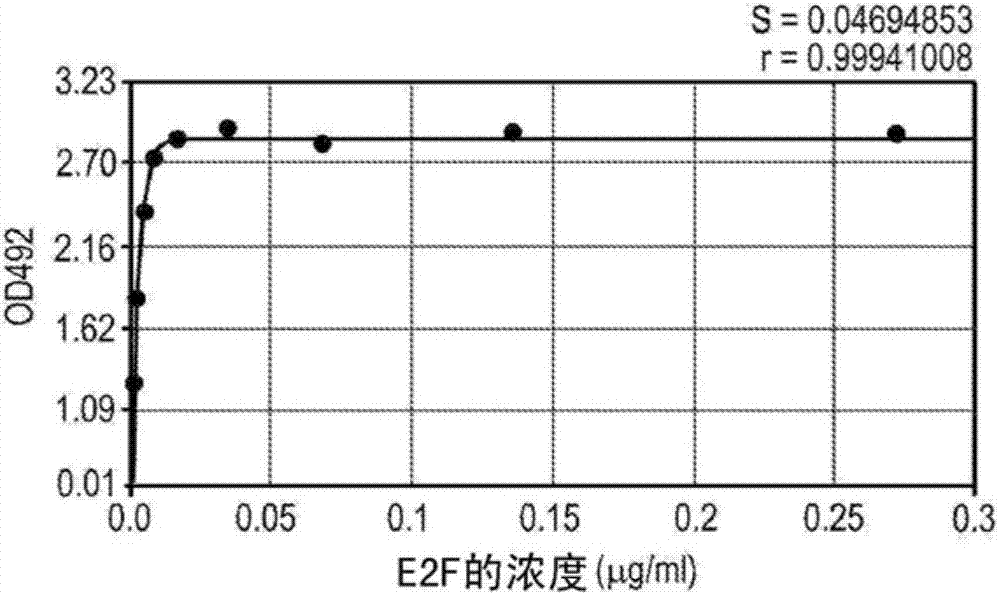
[0107] mAb (2E7) was obtained by immunizing mice with N-acetylmuramoyl-L-alanyl-D-isoglutamine. Antibody isotyping test identifies 2E7 as IgG 1 , and the Kd of 2E7 for N-acetylmuramoyl-L-alanyl-D-isoglutamine was calculated to be 8.7pM ( figure 1 ). By competitive ELISA, it was found that the binding of 2E7 to N-acetylmuramoyl-L-alanyl-D-isoglutamine conjugated to OVA was inhibited in a concentration-dependent manner by muramyl-L-alanyl- Inhibition by D-isoglutamine, N-acetylmuramoyl-L-alanyl-D-glutamate, and muramyl-L-alanyl-D-glutamate was almost identical to that of N- Acetylmuramoyl-L-alanyl-D-isoglutamine was equally effective, suggesting that 2E7 recognizes a common epitope among the four MDPs. However, 2E7 exhibited no response to muramic acid, N-acetylmuramic acid, N-acetylglucosamine, alanine, D-isoglutamine, glutamic acid, glucose, or the 20 common amino acids in proteins Detectable affinity for any one o...
Embodiment 2-2E7
[0108] Determination of the Amino Acid Sequences of Embodiment 2-2E7 Heavy Chain and Light Chain Variable Region
[0109] Messenger RNA was prepared from a 2E7-producing hybridoma clone, which was then used as a template to generate complementary DNA. Variable sequences encoding heavy and light chains, respectively, were amplified by polymerase chain reaction (PCR) using oligonucleotide primer pairs (Table 1) that specifically targeted conserved sequence motifs flanking the coding regions of the variable regions. The DNA fragment of region (this method is described in Kettleborough et al., 1993, Optimization of primers for cloning libraries of mouseimmunoglobulin genes using the polymerase chain reaction. Eur J Immunol 23,206-211 and Pope et al., 1996, Construction of use of antibodies. generepertoire In Antibody Engineering-A Practical Approach. Edited by McCafferty J. Hoogenboom H, and Chiswell D., the contents of both are incorporated herein by reference). The PCR product ...
Embodiment 3
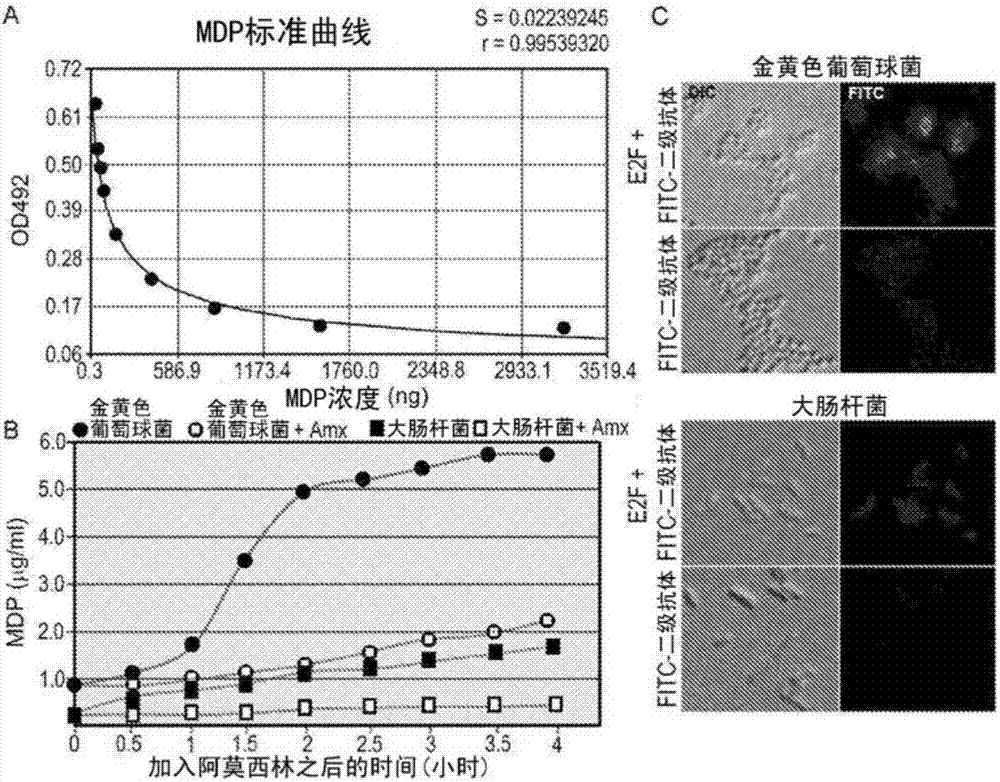
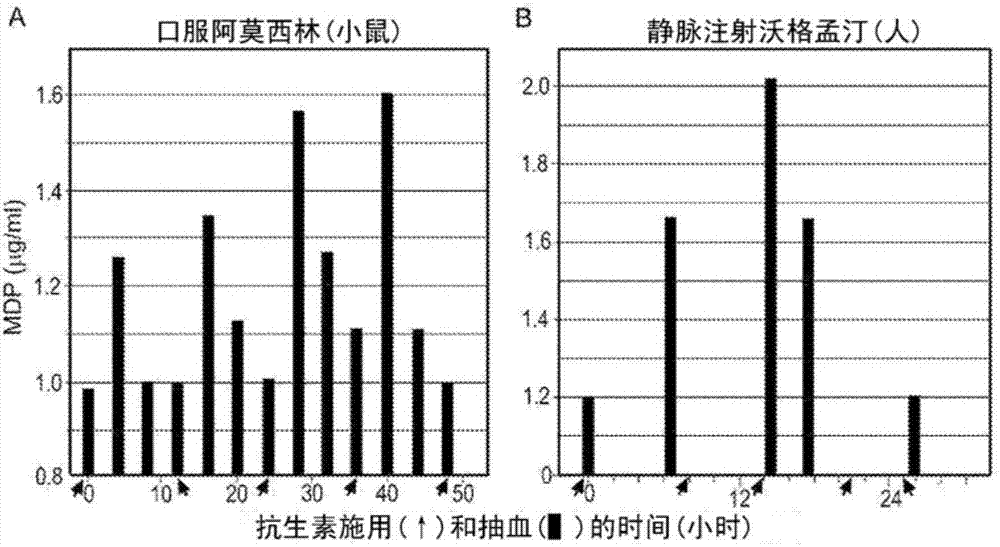
[0116] Example 3 - 2E7 detection of bacterial peptidoglycan in culture medium and on cell surface
[0117] To demonstrate the utility of 2E7, the antibody was first tested for its ability to detect MPs normally present in bacterial cultures. It has been established that β-lactam antibiotics inhibit peptidoglycan polymerization by causing cells to accumulate and secrete MP. The beta-lactam antibiotic amoxicillin (a drug often used in hospitals) was added to the culture and was expected to inhibit 2E7 binding to N-acetylmuramoyl-L-alanyl-D-isoglutamine material increased significantly. For this test, the Gram-positive bacteria Staphylococcus aureus (which has a thick peptidoglycan layer) and Gram-negative E. Grow without amoxicillin. Cultures grown to OD 600 = 1.5 density, then divided into two equal parts. For one aliquot, amoxicillin was added to a final concentration of 40 μg / ml, while for the other aliquot, no drug was added. Both cultures were allowed to continue grow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com