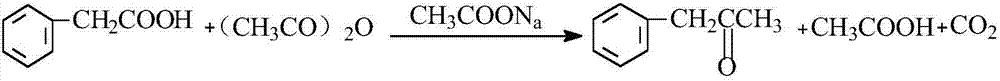Method for preparing phenylacetone from phenylacetic acid
A technology of phenylacetone and phenylacetic acid, applied in the field of preparation of phenylacetone, can solve the problems of low purity of phenylacetone and unreasonable production method of phenylacetone, and achieve the effects of improving reaction, reducing cost and reducing usage amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
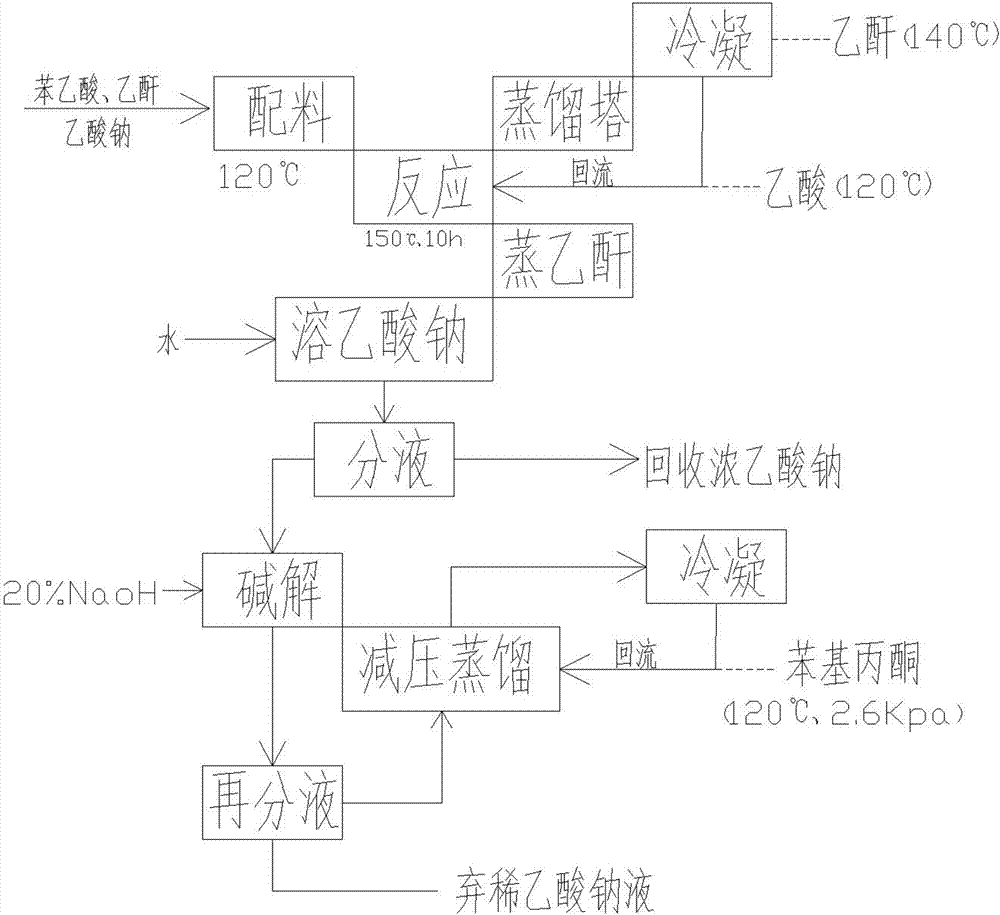
[0026] A method utilizing phenylacetic acid to prepare phenylacetone, comprising the following steps:
[0027] a. Add phenylacetic acid, sodium acetate and acetic anhydride to the enamel reaction kettle in sequence and stir evenly. The molar ratio between sodium and acetic anhydride is 2:1:6;
[0028] b. After stirring phenylacetic acid, sodium acetate and acetic anhydride evenly, put hot oil at 150°C into the jacket of the enamel reaction kettle until acetic acid distills from the top of the distillation tower, and then keep the temperature at the top of the distillation tower at 117°C , use a plastic snake tube to condense the distilled material in the distillation tower and return it to the enamel reaction kettle for 9-11 hours to obtain the reaction liquid;
[0029] c. After the reaction in step b, the reaction solution is heated to distill acetic acid at a temperature of 117° C., and then the temperature is raised to 140° C. to distill acetic anhydride;
[0030] d, cool...
Embodiment 2
[0035] A method utilizing phenylacetic acid to prepare phenylacetone, comprising the following steps:
[0036] a. Add phenylacetic acid, sodium acetate and acetic anhydride to the enamel reaction kettle in sequence and stir evenly. The molar ratio between sodium and acetic anhydride is 2:1:6;
[0037] b. After stirring phenylacetic acid, sodium acetate and acetic anhydride evenly, pour 140°C hot oil into the jacket of the enamel reaction kettle until acetic acid is distilled from the top of the distillation tower, and then keep the temperature at the top of the distillation tower at 120°C , using a plastic snake tube to condense the distilled material in the distillation tower and return it to the enamel reaction kettle for 10 hours to react to obtain a reaction liquid;
[0038] c. After the reaction in step b, the reaction solution is heated to distill acetic acid at a temperature of 120° C., and then the temperature is raised to 140° C. to distill acetic anhydride;
[0039...
Embodiment 3
[0044] A method utilizing phenylacetic acid to prepare phenylacetone, comprising the following steps:
[0045] a. Add phenylacetic acid, sodium acetate and acetic anhydride to the enamel reaction kettle in sequence and stir evenly. The molar ratio between sodium and acetic anhydride is 2:1:6;
[0046] b. After stirring phenylacetic acid, sodium acetate and acetic anhydride evenly, put hot oil at 145°C into the jacket of the enamel reaction kettle until acetic acid is distilled from the top of the distillation tower, and then keep the temperature at the top of the distillation tower at 117°C , use a plastic snake tube to condense the distilled material in the distillation tower and return it to the enamel reaction kettle for 9-11 hours to obtain the reaction liquid;
[0047]c. After the reaction in step b, the reaction solution is heated to distill acetic acid at a temperature of 118° C., and then heated to 145° C. to distill acetic anhydride;
[0048] d, cooling the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com