Anthraquinone compound, and preparation method and application thereof
A compound, anthraquinone technology, applied in the field of phytochemistry, can solve problems such as no relevant reports, achieve good antioxidant activity, easy to implement, and prolong the shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The aloe sample was collected in Yuanjiang, Yunnan. 3.5kg of dried aloe was taken, coarsely crushed to 30 mesh, ultrasonically extracted with 70% acetone for 4 times, 60 minutes each time, and the extracts were combined; the extracts were filtered and concentrated under reduced pressure to 1% of the volume. / 4; leave standstill, filter out the precipitate, and concentrate into 110g of extract; add 250g of acetone to dissolve in the extract, then add 100 mesh silica gel 120g to mix the sample, after mixing the sample, pack the column with 200 mesh silica gel 600g; The volume ratios were 1:0, 9:1, 8:2, 7:3, 1:1, and 0:1 for gradient elution with chloroform-acetone mixed organic solvents, and the gradient eluate was collected, concentrated, and monitored by TLC. Merge the same parts to get 6 parts A-F, among them, for the collected sample D (7:3) part 18g, then use 48% methanol as mobile phase, flow rate 20ml / min, 21.2×250mm, 5μm Zorbax PrepHT The GF reversed-phase prepara...
Embodiment 2
[0040] The aloe sample was collected in Hekou, Yunnan Province, and 4.2kg of dried aloe was taken, coarsely crushed to 35 mesh, ultrasonically extracted 4 times with 70% acetone, 50 minutes each time, and the extracts were combined; the extracts were filtered and concentrated under reduced pressure to 1% of the volume. / 4; leave standstill, filter out the precipitate, and concentrate into 136g of extract; add 280g of acetone to dissolve in the extract, then add 100 mesh silica gel 140g to mix the sample, after mixing the sample, pack the column with 200 mesh silica gel 900g; The volume ratios were 1:0, 9:1, 8:2, 7:3, 1:1, and 0:1 for gradient elution with chloroform-acetone mixed organic solvents, and the gradient eluate was collected, concentrated, and monitored by TLC. Merge the same parts to get 6 parts A-F, among them, for the collected sample D (7:3) part 22g, then use 48% methanol as mobile phase, flow rate 20ml / min, 21.2×250mm, 5μm Zorbax PrepHT The GF reversed-phase pr...
Embodiment 3
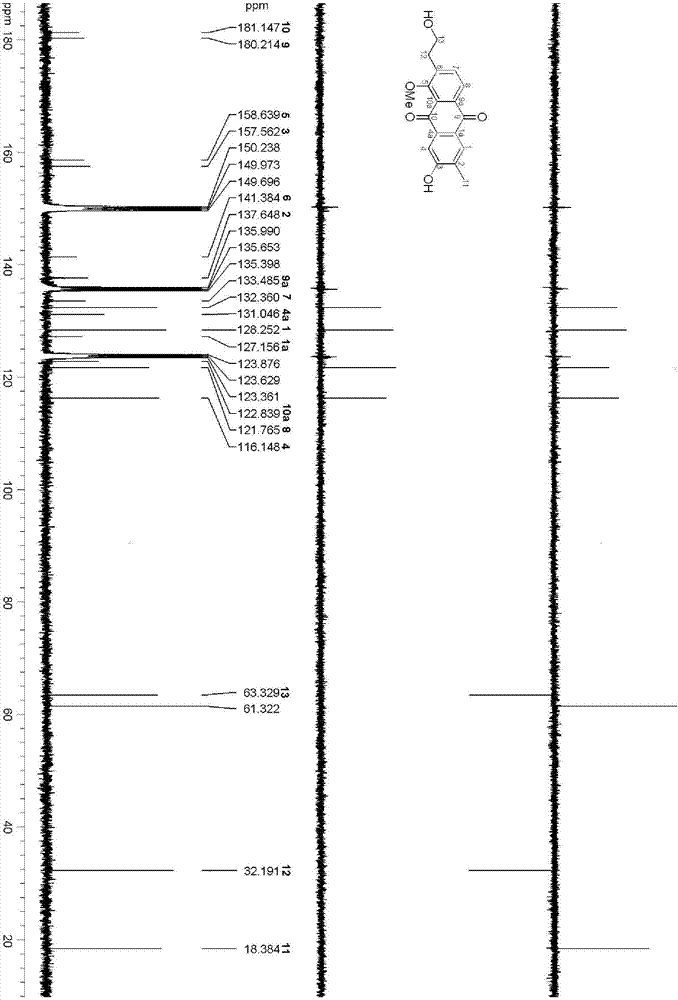
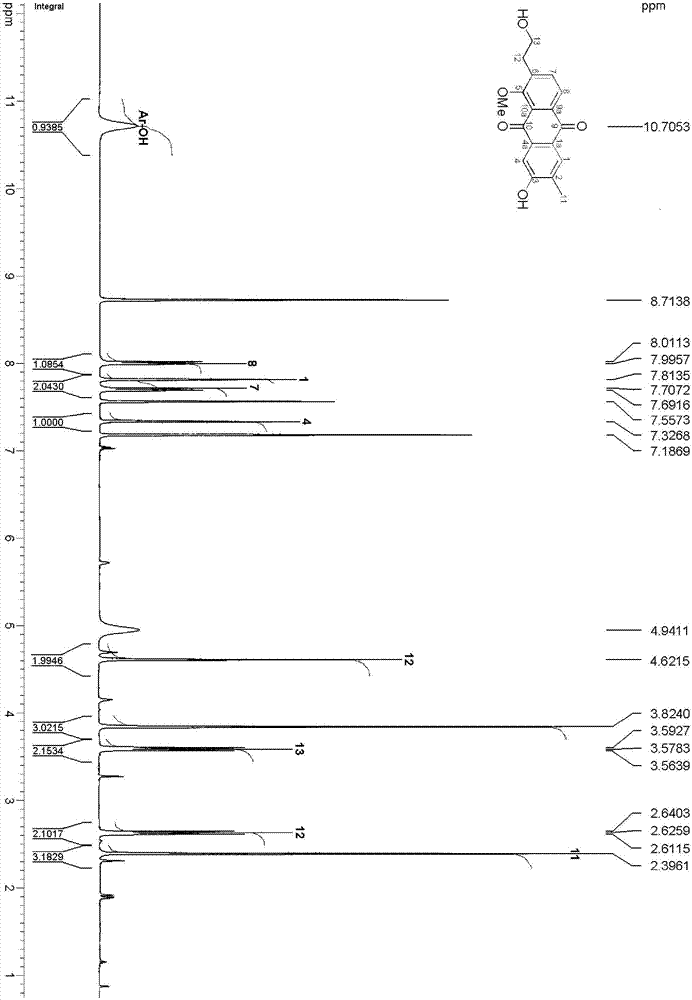
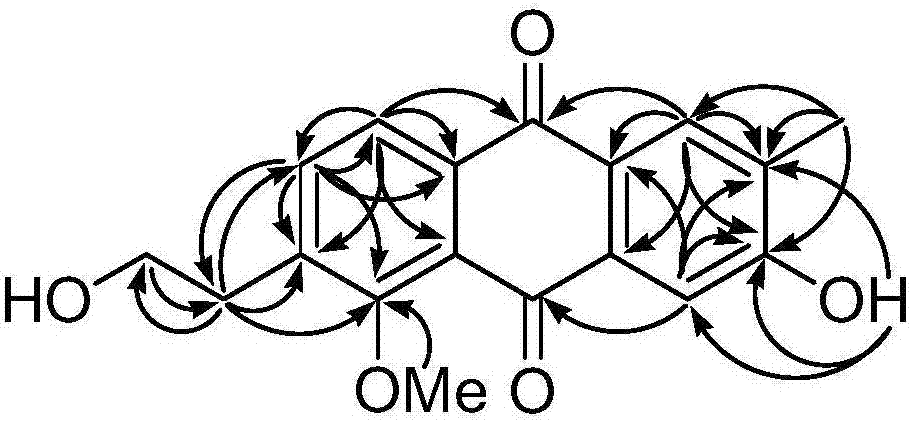
[0042] The structure of the anthraquinone compounds prepared in Example 1 is determined by the following method; the compound is a red jelly; HRESI-MS shows that its quasi-molecular ion peak is 335.0887[M+Na] + (calculated value 335.0895), combined with 1 HNMR and DEPT spectra confirmed that its molecular formula is C 18 h 16 o 5 , with an unsaturation of 11. The hydroxyl group (3407cm ‐1 ), carbonyl (1662cm ‐1 ) and aromatic rings (1617, 1554 and 1448cm ‐1 ) resonance absorption peak. The UV spectrum has maximum absorption at 210, 248, 276 and 407nm, which also shows that there may be an aromatic ring structure in the compound. compound 1 H and 13 The C NMR spectrum (Table-1) shows that it contains 17 carbons and 14 hydrogens, including two benzene rings (C1-C8, C-1a, C-4a, C-9a and C-10a; H-1, H -4, H-7 and H-8), two carbonyl groups (C-9 and C-10), one hydroxyethyl group (C-12 and C-13; H 2 ‐12 and H 2 ‐13), a methyl group (C‐11; H 3 ‐11), a methoxy group (δ C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com