Microfluidic devices with smooth surfaces for the enrichment of rare cells and biomarkers from biological fluids
A microfluidic device and biofluidic technology, applied in animal cells, fluid controllers, laboratory containers, etc., can solve the problems of low flow rate, reduced fluid volume, and insufficient rapid processing of a large amount of blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
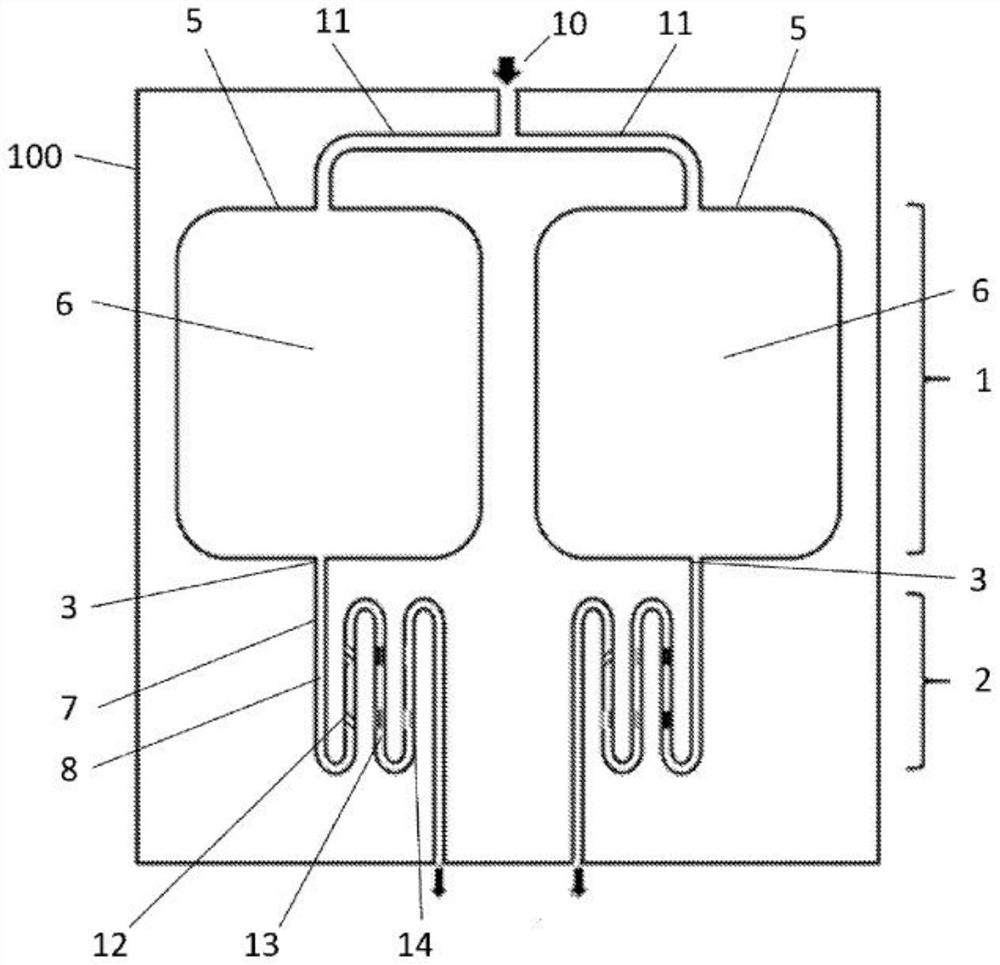
[0063] Improved microfluidic devices for handling rare whole cells (eg, circulating tumor cells) are disclosed. Improvements include 1) smooth surface design in the cell capture module; 2) combined cell and biomarker capture mechanism; and, 3) "multiplex" capture of different biomarkers in separate bands in the cell-free biomarker module .
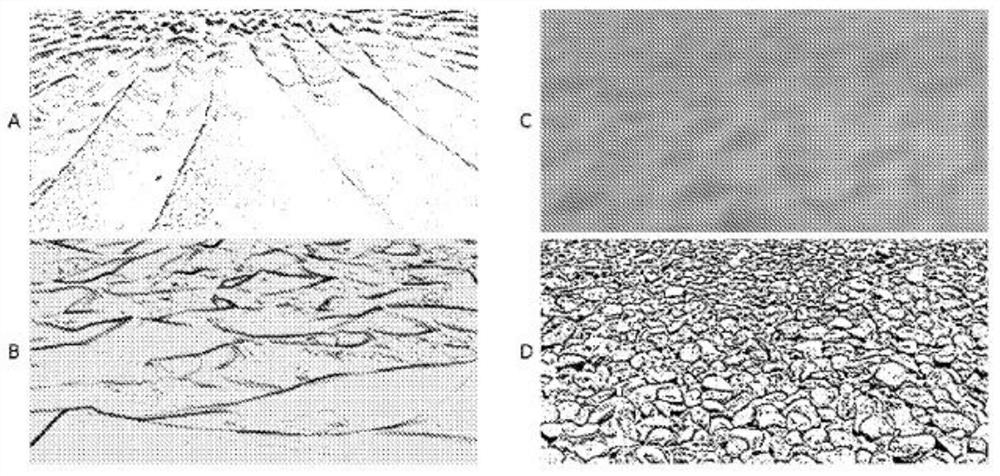
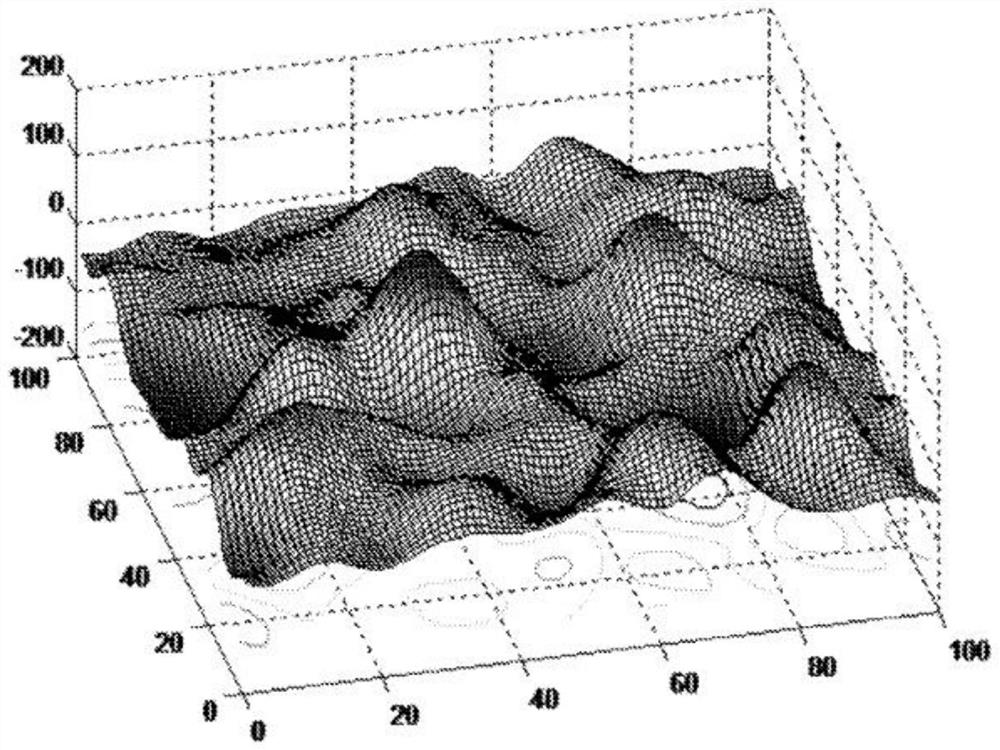
[0064] In summary, the microfluidic device has two integrated capture modules. The first capture module includes a capture zone with an inner surface. The bottom of the inner surface of the first capture module includes a micrometer (μm) to millimeter (mm) level seabed or riverbed simulated smooth topography that facilitates cell-device inner surface interactions. The above-mentioned smooth shape is simulated by using Gaussian or other smooth mathematical functions. Smooth features create a gentle environment to enhance sample mixing, continuous cell-antibody contact, and rare cell capture. The smooth surface design also reduces the sh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com