Application of anti-adhesion polypeptide, implanted medical instrument and preparation method thereof
A technology for implanting medical devices and adhesion, applied in medical science, coating, surgery, etc., can solve the problems of hyperplasia, poor specificity, etc., and achieve the effects of inhibiting inflammatory reactions, improving efficiency, and improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Cell experiment
[0067] To verify the adhesion performance of the cells treated with the anti-cell adhesion polypeptide on the surface of the culture plate.
[0068] (1) Cell culture
[0069] Human arterial smooth muscle cells were cultured in DMEM (Gibico brand) high glucose medium containing 10% FBS (Gibico brand). Human umbilical vein endothelial cells cultured in EGM TM -2BulletKit TM (Lonza, CC-3162) medium containing 10% FBS and various growth factors.
[0070] (2) Cell inoculation
[0071] When the number of cells reaches 80% of the bottom area of the culture flask, suck off the medium and add trypsin. When the cells shrink from the bottom of the culture flask but do not completely detach, suck off the trypsin and tap the bottom of the flask until the cells are completely detached from the bottom of the flask. Add 10 mL of PBS solution containing 1% FBS to stop the digestion, and pipette the cells to form a cell suspension. Aliquot 10mL of the...
Embodiment 2
[0077] Embodiment 2 animal experiments
[0078] To verify the effect of anti-adhesion peptides on cell-trapping scaffolds to promote endothelial repair.
[0079] (1) Sample preparation
[0080] (1) Antibody loading
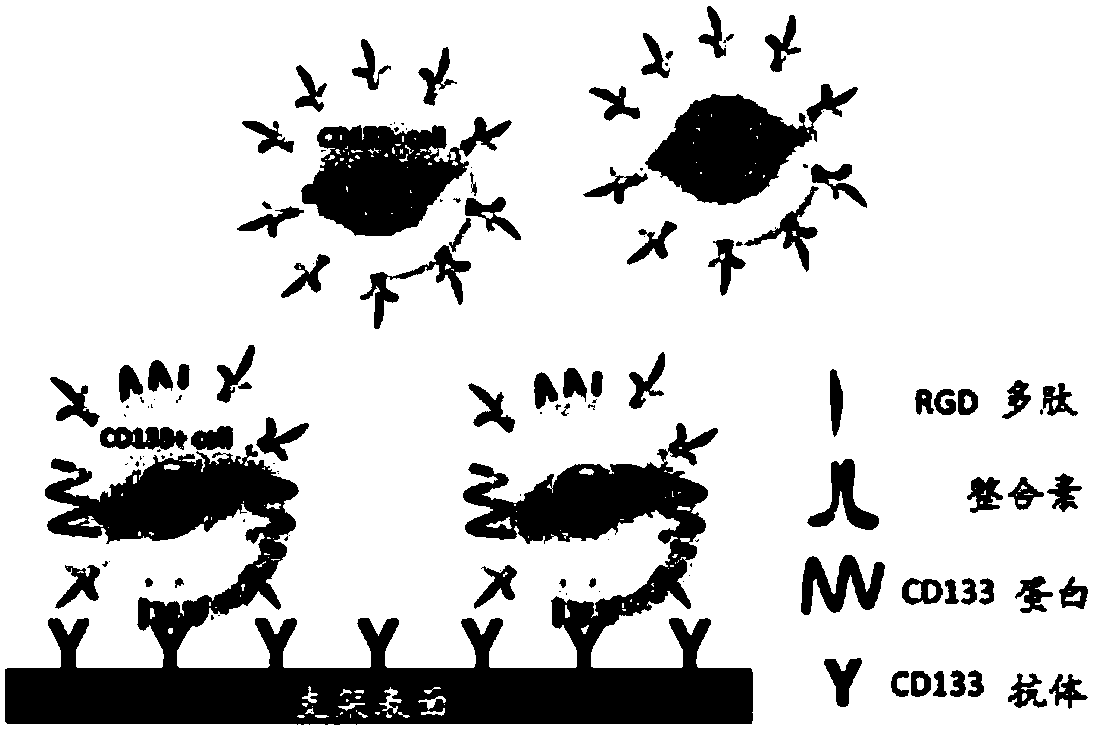
[0081] Prepare PEI solution (5mg / mL, 0.15M NaCl), chitosan solution (CS, 1mg / mL, 0.15M NaCl, pH=4.0), sodium hyaluronate solution (HA, 1mg / mL, 0.15M NaCl, pH =6.0), NaCl solution (0.15M), AC133 (CD133-1) antibody solution 1g / mL.
[0082] Cut the L605 bare metal bracket into a structure with a hole in the bracket rod. with H 2 SO 4 :H 2 o 2 (30%)=3:1 solution for 30 minutes, followed by deionized ultrasonic cleaning for 3 times, 10 minutes each time. After being blown dry with nitrogen gas, first dip it in polyethyleneimine (PEI) solution, let it stand for 30 minutes, take it out, wash it, and blow it dry. Soak the PEI-treated stent in sodium hyaluronate solution, wash and dry after standing for 20 minutes, and obtain the outer layer of HA; then soak the s...
Embodiment 3
[0095]Prepare PEI solution (5mg / mL, 0.15M NaCl), chitosan solution (CS, 1mg / mL, 0.15M NaCl, pH=4.0), sodium hyaluronate solution (HA, 1mg / mL, 0.15M NaCl, pH =6.0), NaCl solution (0.15M), cell capture substance AC133 (CD133-1) antibody solution 10 mg / mL.
[0096] Cut the L605 bare metal bracket into a structure with a hole in the bracket rod. with volume ratio H 2 SO 4 :H 2 o 2 (30%) = 3:1 solution washing for 30 minutes, washing with pure water, and drying with nitrogen. Then soak it in PEI solution, take it out after 30 minutes, wash and dry it. Soak the PEI-treated stent in sodium hyaluronate solution, wash and dry after standing for 20 minutes, and obtain the outer layer of HA; then soak the stent in chitosan solution, wash and dry after standing for 20 minutes, The CS outer layer was obtained; the above process was repeated 7 times to obtain a sodium hyaluronate / chitosan self-assembled 7-layer film. Soak the scaffold in the CD133-1 (AC133) antibody solution, and let...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com