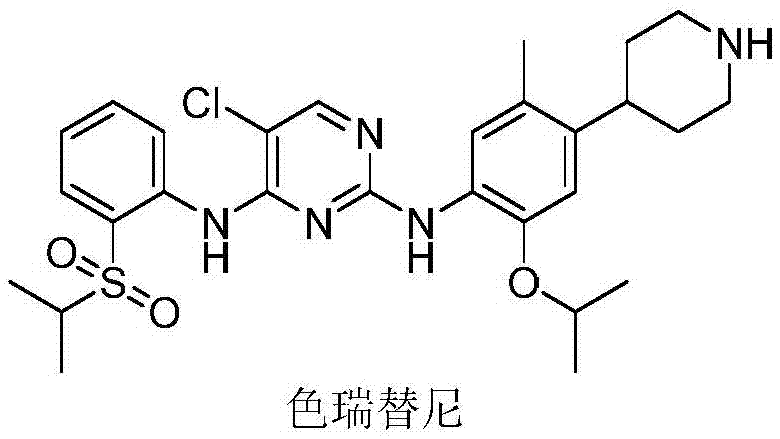
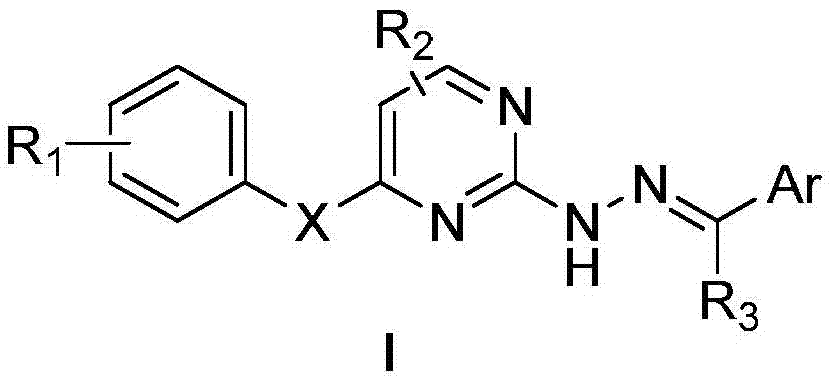
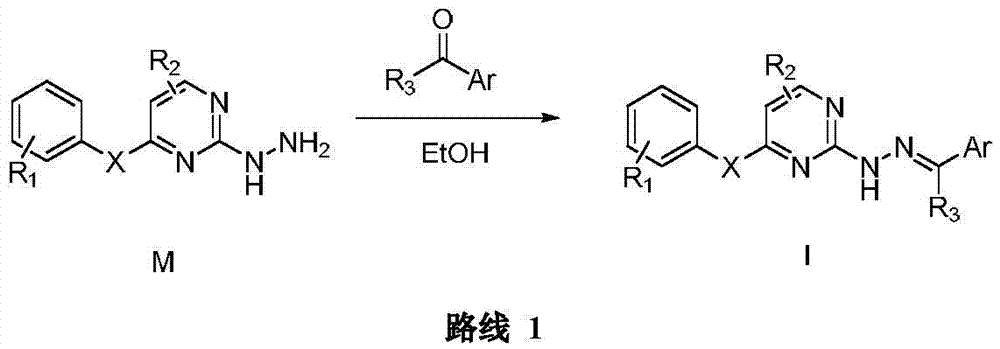
Hydrazone-containing pyrimidine derivatives and use thereof
A technology of derivatives and compounds, applied in the field of hydrazone-containing pyrimidine derivatives and their uses, can solve problems such as ROS1 protein dysregulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Step A Isopropyl(2-nitrophenyl)sulfide (Ⅱ)
[0116] Add 509.1g (3.61mol) of o-fluoronitrobenzene and 698.0g (5.06mol) of anhydrous potassium carbonate to 2500mL of dry N,N-dimethylformamide (DMF), slowly drop into 301.8g (3.97mol ) isopropyl mercaptan, after dropping, the temperature was raised to 110° C. for 10 h. After cooling to room temperature, the reaction solution was poured into a large amount of water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain 604.6 g of a yellow liquid with a yield of 85.0%.
[0117] Step B 1-Isopropylsulfonyl-2-nitrobenzene (Ⅲ)
[0118] Add 197.1g (1.00mol) of isopropyl (2-nitrophenyl) sulfide to 1500mL of glacial acetic acid, slowly drop into 583.0g (6.00mol) of 35% hydrogen peroxide, raise the temperature to 80°C, and react for 9h. After cooling to room temperature, the reaction solution was poured into a large amount of water, stirred at room temperature for 30 min, filtered wi...
Embodiment 2
[0129] Example 2 (E)-5-chloro-N-(2-(isopropylsulfonyl)phenyl)-2-(2-(pyridin-4-ylmethylene)hydrazino)pyrimidin-4-amine
[0130] ESI-MS [M+H] (m / z): 431.1; 1 H NMR(400MHz,DMSO)δ11.69(s,1H),9.80(s,1H),9.21(s,1H),8.36(s,1H),8.10(s,1H),7.88(t,J= 7.8Hz, 2H), 7.64(d, J=5.8Hz, 2H), 7.40(t, J=7.6Hz, 1H), 3.58–3.45(m, 1H), 1.20(d, J=6.8Hz, 6H) .
Embodiment 3
[0131] Example 3 (E)-5-chloro-N-(2-(isopropylsulfonyl)phenyl)-2-(2-(pyridin-3-ylmethylene)hydrazino)pyrimidin-4-amine
[0132] ESI-MS [M+H] (m / z): 431.1;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com