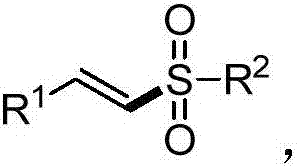
(E)-alkenyl sulfone compound and preparation method thereof
A compound, alkenyl sulfone technology, applied in the field of alkenyl sulfone compounds and their preparation, can solve the problems of poor regioselectivity and stereoselectivity, cumbersome production, and many steps, and achieve high yield, good substrate compatibility, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
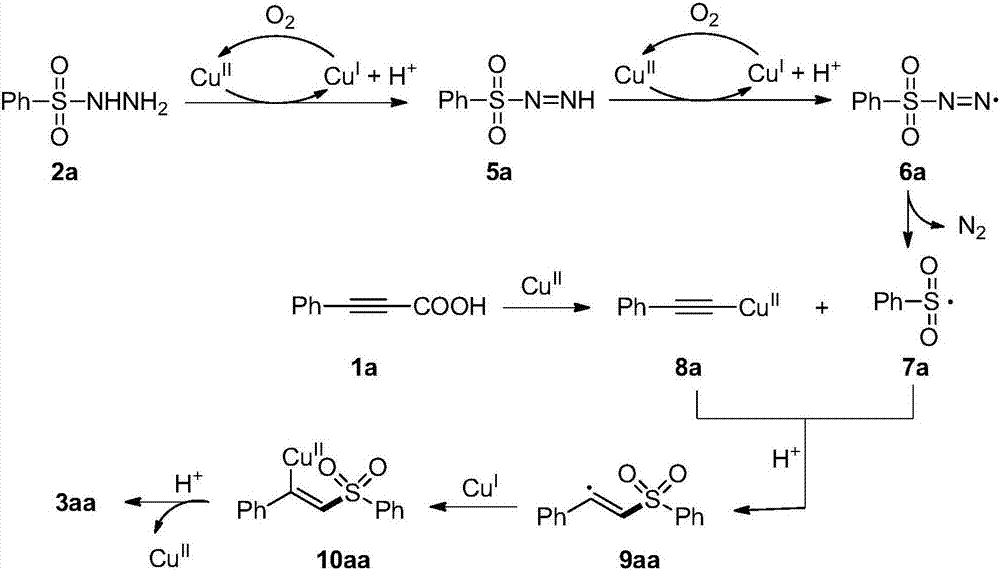
[0028] Transition metal copper catalyzed synthesis of alkenyl sulfone from phenylpropiolic acid and phenylsulfonyl hydrazide with respect to catalyst, solvent and reaction time. Where [Cu] is the copper catalyst, S is the organic solvent, and T is the reaction temperature. After screening the reaction conditions, we got the optimal reaction conditions, THF was the solvent, Cu 2 O is the catalyst, the temperature is 80°C, the reaction time is 24h, and oxygen in the air is used as the oxidant.
[0029]
[0030]
[0031]
[0032] in a for 1 H NMR yield, 18 b For the reaction gas to N 2 gas.
Embodiment 2
[0034] Copper-catalyzed synthesis of alkenyl sulfones from phenylpropiolic acid series substrates and phenylsulfonyl hydrazides. Through the extended research on phenylpropiolic acid substrates, it is found that phenylpropiolic acid with different substituent groups can be applied to this method and obtain moderate to good yields.
[0035]
[0036] The specific experimental operation is to add phenylpropiolic acid substrate (0.02mmol), phenylsulfonyl hydrazide (0.04mmol) and THF (2.0mL) in sequence in a dry and clean reaction tube. React in 24h. After the reaction, the organic phase was extracted, and after the solvent was removed under reduced pressure, the target product 3 was obtained by thin-layer chromatography, as follows:
[0037] Target product 3ba: (E)-1-methyl-4-(2-(phenylsulfonyl)vinyl)benzene
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.94(d, J=8.0Hz, 2H), 7.66(d, J=15.4Hz, 1H), 7.63-7.51(m, 3H), 7.38(d, J=8.0Hz, 2H), 7.19( d,J=8.0Hz,2H),6.80(d,J=15.4Hz...
Embodiment 3
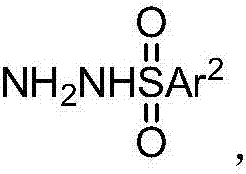
[0067] Copper-catalyzed synthesis of alkenyl sulfones from phenylpropiolic acid and phenylsulfonyl hydrazide series substrates. Through the extended research on phenylsulfonyl hydrazide substrates, it is found that the method is suitable for phenyl sulfonyl hydrazides substituted by electron-withdrawing groups or electron-deficient groups, as well as phenyl sulfonyl hydrazides with large steric hindrance effect. , and good yields can also be obtained.
[0068]
[0069] The specific experimental operation is to add phenylpropiolic acid (0.02mmol), phenylsulfonyl hydrazide series substrates (0.04mmol) and THF (2.0mL) in sequence in a dry and clean reaction tube. React in 24h. After the reaction, the organic phase was extracted, and after the solvent was removed under reduced pressure, the target product 3 was obtained by thin-layer chromatography, as follows:
[0070] Target product 3aa: (E)-1-Phenylsulfonyl-2-phenylethene
[0071]
[0072] 1 H NMR (400MHz, CDCl 3 )δ7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com