Synthesis method of amino acid quaternary amino carboxylate
A kind of technology of quaternary amino carboxylate and synthesis method, applied in the field of compound synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
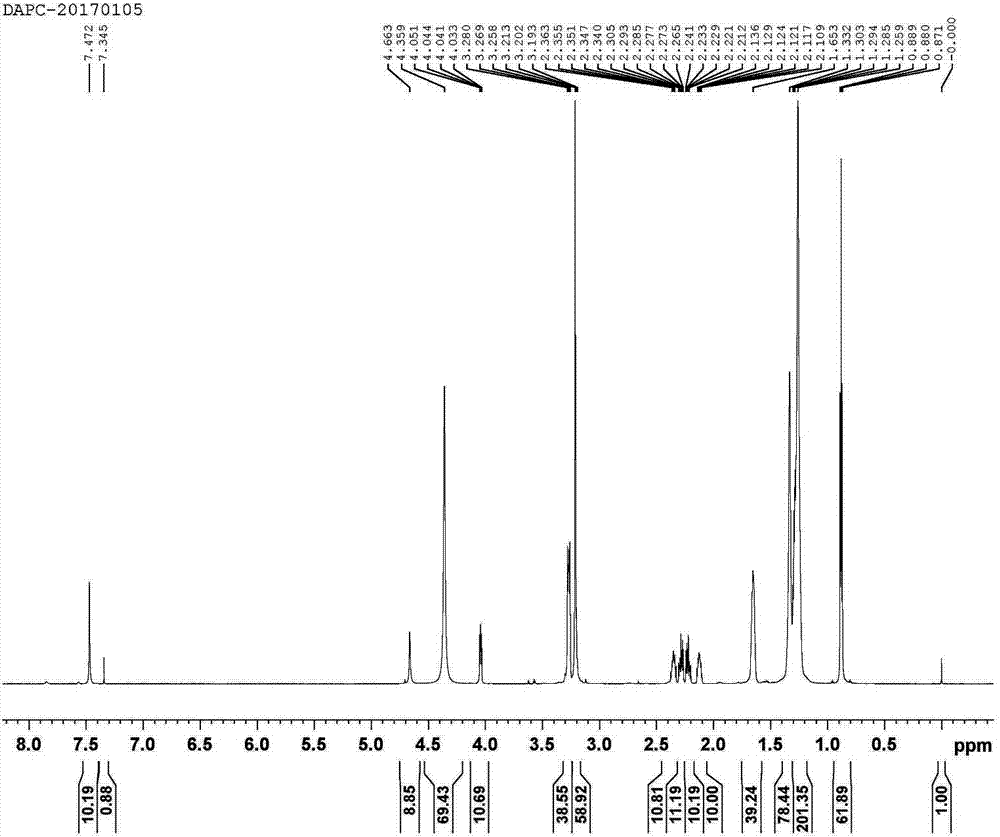
[0060]Synthesis of DAPC (Proline,5-oxo-,ion(1-),N-decyl-N,N-dimethyl-1-decanaminium(1:1)) Proceed as follows:
[0061] Preparation of solution A: Take 10 mL of absolute ethanol, dissolve 0.0024 mol of KOH in it, heat to 30°C, stir well until KOH is completely dissolved, and obtain solution A;
[0062] Preparation of solution B: Take 10 mL of absolute ethanol, add 0.0024 mol of didecyldimethylammonium chloride into it at room temperature, stir well until the didecyldimethylammonium chloride is completely dissolved, and obtain solution B;
[0063] Preparation of solution C: Mix solution A and solution B at room temperature and stir for 5 minutes to form a precipitate of potassium chloride insoluble in absolute ethanol, fully filter or centrifuge to remove the precipitate of potassium chloride to obtain double decyl dimethyl ammonium hydroxide solution, to obtain solution C;
[0064] Preparation of solution D: Dissolve 0.0024mol of pyroglutamic acid in absolute ethanol to make ...
Embodiment 2
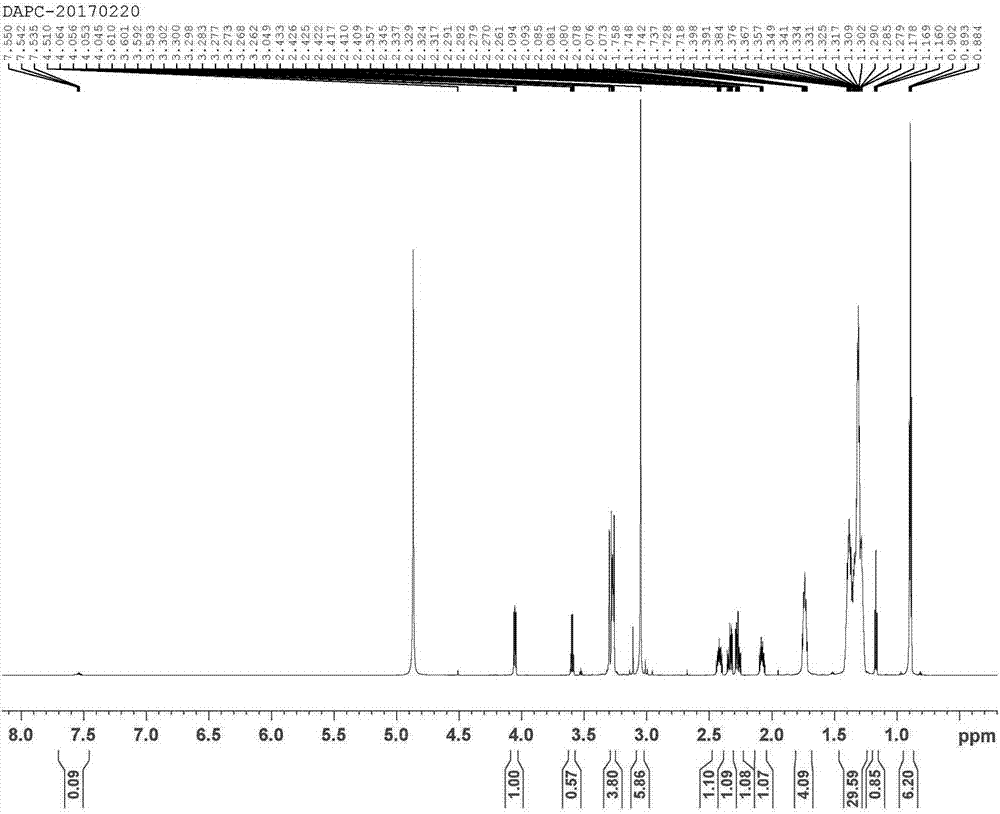
[0069] Synthesis of DAPC (Proline,5-oxo-,ion(1-),N-decyl-N,N-dimethyl-1-decanaminium(1:1)) Proceed as follows:
[0070] Prepare solution A: Take 20 mL of absolute ethanol, dissolve 0.005 mol of KOH in it, heat to 30°C, and stir well until KOH is completely dissolved to obtain solution A;
[0071] Preparation of solution B: Take 15 mL of absolute ethanol, add 0.005 mol of didecyldimethylammonium chloride into it at room temperature, stir well until the didecyldimethylammonium chloride is completely dissolved, and obtain solution B;
[0072] Preparation of solution C: Mix solution A and solution B at room temperature and stir for 5 minutes to form a precipitate of potassium chloride insoluble in absolute ethanol, fully filter or centrifuge to remove the precipitate of potassium chloride to obtain double decyl dimethyl ammonium hydroxide solution, to obtain solution C;
[0073] Preparation of solution D: Dissolve 0.005mol of pyroglutamic acid in absolute ethanol to make a solut...
Embodiment 3
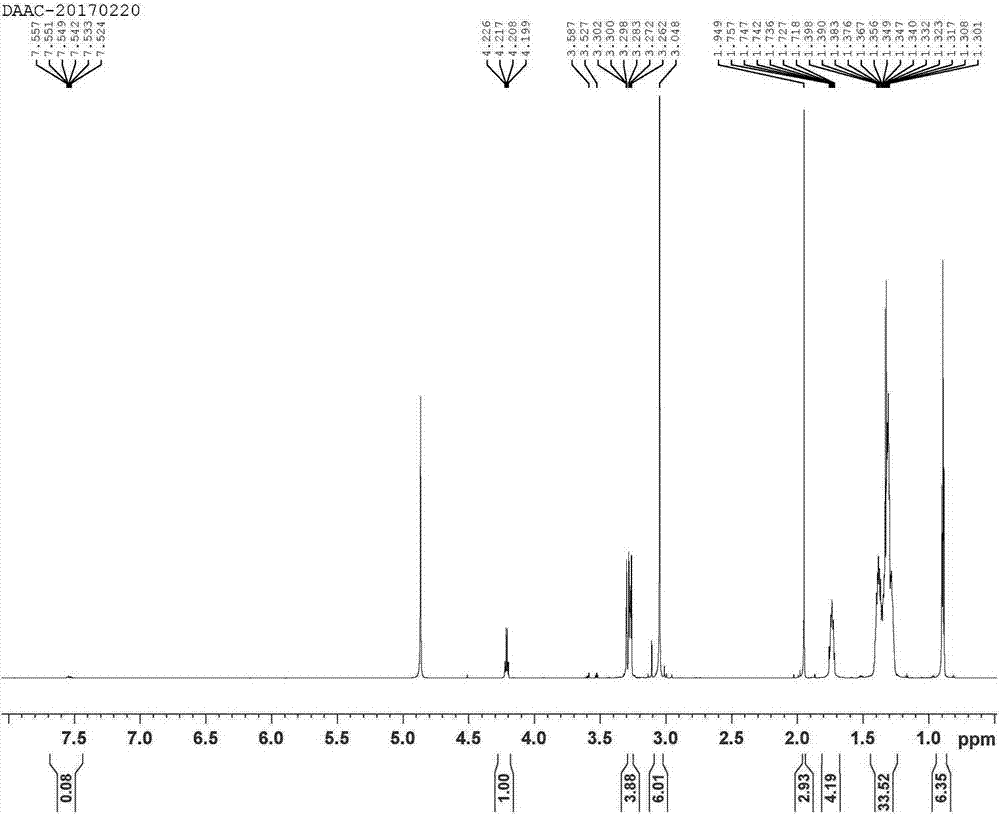
[0078] The synthetic amino acid double-chain quaternary amino carboxylate is double-decyl dimethyl N-acetylalanine ammonium DAAC (1-Decanaminium, N-decyl-N, N-dimethyl-, salt with N-acetylalanine (1:1 ))
[0079] Preparation of solution A: Dissolve 0.0025mol of KOH in absolute ethanol as a solvent, heat to 20°C-30°C, and stir well until KOH is completely dissolved to obtain solution A;
[0080] Prepare solution B: use absolute ethanol as a solvent, add 0.0025mol of didecyldimethylammonium chloride into it at room temperature, stir well until the didecyldimethylammonium chloride is completely dissolved, and obtain solution B ;
[0081] Preparation of solution C: Mix solution A and solution B at room temperature, stir for 1 to 10 minutes, generate potassium chloride precipitate insoluble in absolute ethanol, fully filter or centrifuge to remove potassium chloride precipitate, double decyl dimethyl hydroxide Ammonium solution, to obtain solution C;
[0082] Preparation of solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com