Histone demethylase LSD1 (lysine specific demethylase 1) inhibitor
An aryl and hydroxyl technology, which is applied in the field of histone lysine demethylase LSD1 inhibitors, can solve the problems that LSD2 cannot be found, proteins cannot form complexes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
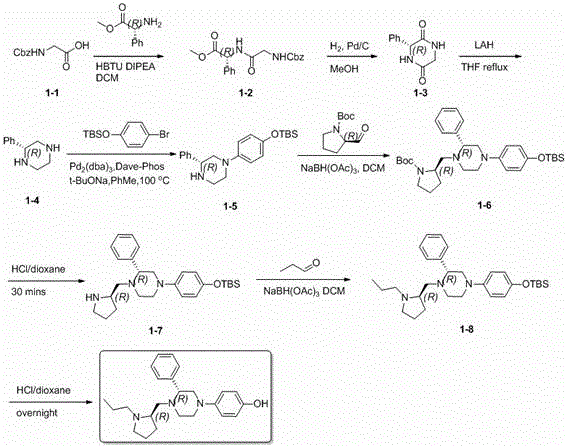
[0081] The preparation of the derivative (compound-1) shown in embodiment 1 general formula (I)
[0082] 4-((R)-3-phenyl-4-(((R)-1-propylpyrrolin-2-yl)methylene)piperazin-1-yl)phenol (hereinafter referred to as compound 1) preparation
[0083]
[0084] Compound-1
[0085] This compound can be prepared by the following steps:
[0086]
[0087] 1.1 Preparation of (R)-2-(2-(benzyloxycarbonylamino)acetamido)-2-phenylacetic acid methyl ester (1-2)
[0088]
[0089] 2-Benzyloxycarbonylaminoacetic acid (1-1) (2.98 g) and R-2-amino-2-phenylacetic acid methyl ester hydrochloride (3.0 g, 14.3 mmol) were suspended in anhydrous dichloromethane (30 mL), HBTU (6.66 g) and DIEA (7.4 g, 57.4 mmol) were added. After the reaction mixture was stirred overnight at room temperature, water (10 mL) was added, and after separation, the organic phase was extracted twice with dichloromethane (10 mL×2), and the combined organic phase was washed once with saturated brine, washed with anhydro...
Embodiment 2
[0112] Example 2 Preparation of derivatives represented by general formula (I) (compound-2)
[0113] Preparation of (R)-4-(3-phenyl-4-(piperidin-4-ylmethylene)piperazin-1-yl)phenol (hereinafter collectively referred to as compound 2)
[0114]
[0115] Compound-2
[0116] This compound can be prepared by the following steps:
[0117]
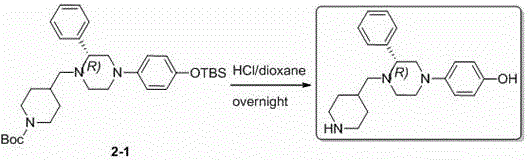
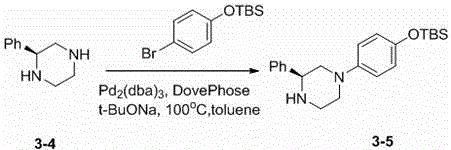
[0118] 2.1 (R)-1-(4-(N-tert-butoxycarbonylpiperidinyl)methylene)-2-phenyl-4-(4-(tert-butyldimethylsilyloxy)phenyl) ) Preparation of piperazine (2-1)
[0119]
[0120] (R)-1-(4-(tert-Butyldimethylsilyloxy)phenyl)-3-phenylpiperazine (1-5) (100 mg) and 1-tert-butoxycarbonylpiperidine-4- Formaldehyde (69 mg) was obtained using the same experimental procedure as in step 1.5 to obtain (R)-1-(4-(N-tert-butoxycarbonylpiperidinyl)methylene)-2-phenyl-4-(4- (tert-butyldimethylsilyloxy)phenyl))piperazine (2-1) 130 mg. LC-MS (ESI): m / z (M+1) 566.4.
[0121] Phenyl-4-(piperidin-4-ylmethylene)piperazin-1-yl)phenol ( Compound 2 ) preparation
[...
Embodiment 3
[0125] The preparation of the derivative (compound-3) shown in embodiment 3 general formula (I)
[0126] Preparation of (S)-4-(4-(4-hydroxybenzyl)-3-phenylpiperazin-1-yl)phenol (hereinafter referred to as compound 3)
[0127]
[0128] Compound-3
[0129] This compound can be prepared by the following steps:
[0130]
[0131] 3.1 Preparation of (S)-2-(2-(benzyloxycarbonylamino)acetamido)-2-phenylacetic acid methyl ester (3-2)
[0132]
[0133]2-Benzyloxycarbonylaminoacetic acid (3-1) (2.98 g) and S-2-amino-2-phenylacetic acid methyl ester hydrochloride (3.0 g) were dissolved in anhydrous DMF (30 mL), and HBTU was added (6.66 g) and DIEA (7.4 g). After the reaction mixture was stirred overnight at room temperature, water (30 mL) and ethyl acetate (10 mL) were added, and after separation, the aqueous phase was extracted with ethyl acetate (10 mL X 3), and the combined organic phase was washed 3 times with brine , dried over anhydrous sodium sulfate, filtered and conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com