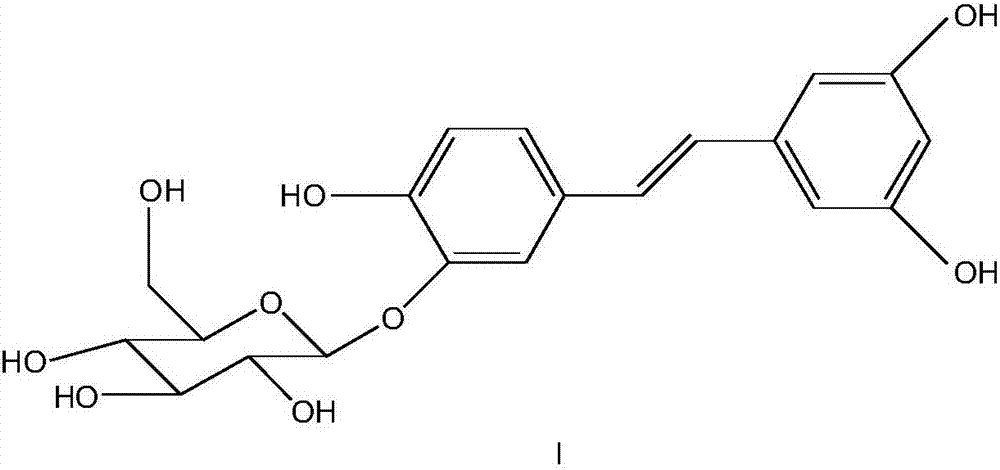
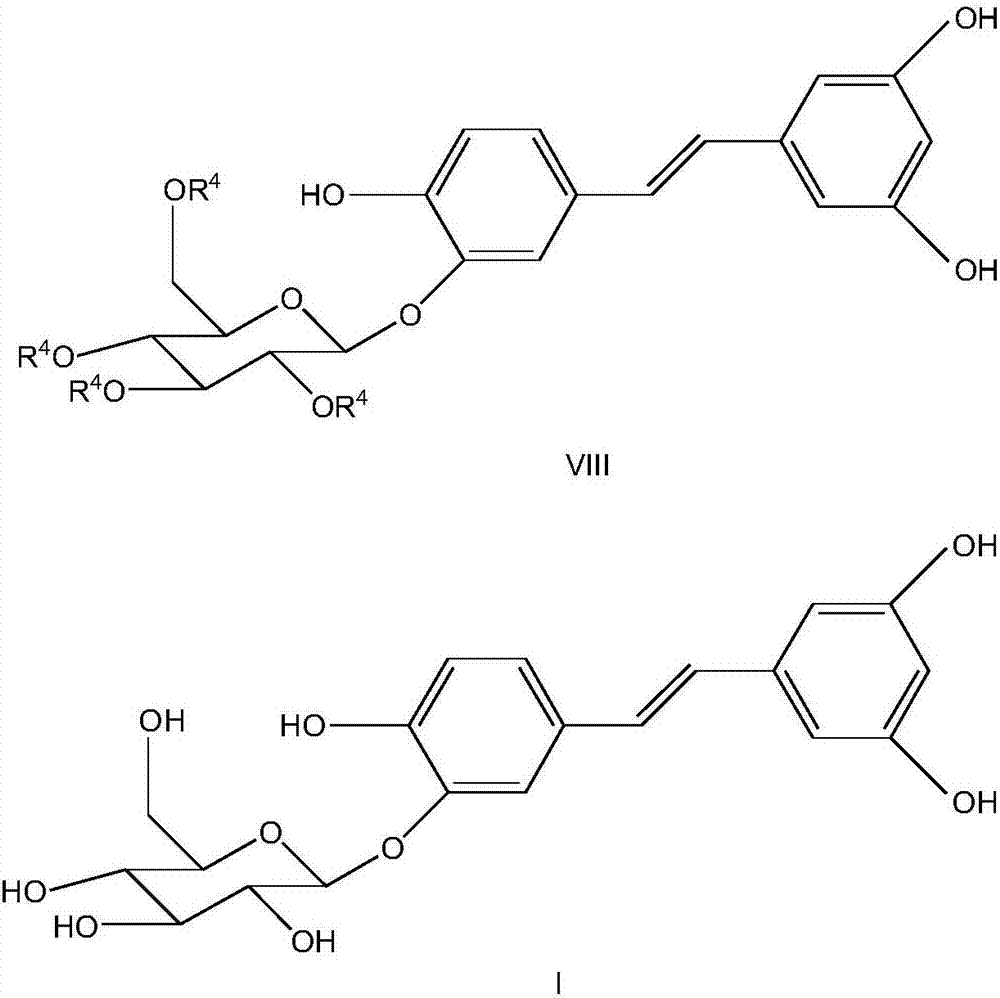
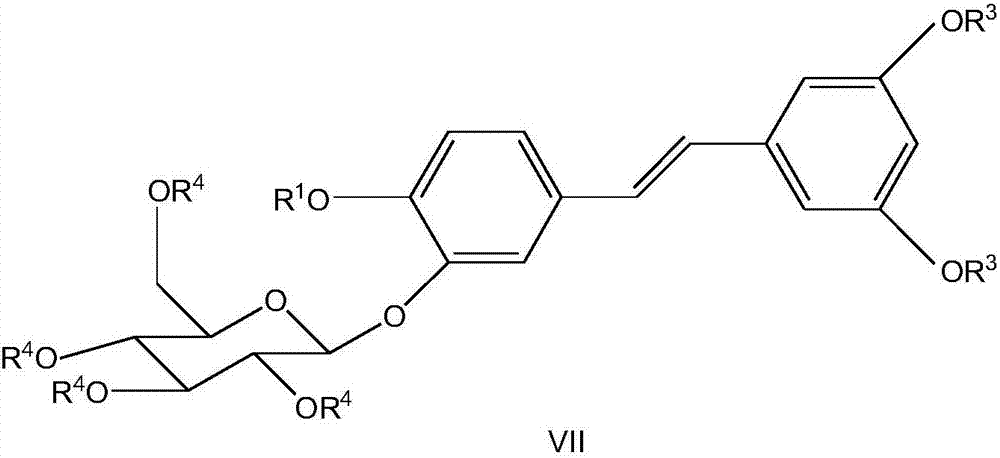
Synthesizing method of piceatannol 3'-O-belta-D-glucopyranoside
A synthesis method and a technique for tristilbene glycosides are applied in the field of compound synthesis and can solve problems such as chemical synthesis of tristilbene glycosides that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1, preparation formula S1 compound
[0105]
[0106] 3,4-Dihydroxybenzaldehyde (5.0 g, 36.2 mmol) was dissolved in N,N-dimethylformamide (100 ml), and sodium carbonate (9.59 g, 90.6 mmol) was added thereto. After stirring at room temperature for 5 minutes, allyl bromide (3.13ml, 36.2mmol) was slowly added dropwise thereto. Continue to stir and react at room temperature for 12 hours. After the reaction is completed, use diatomaceous earth to filter and collect the filtrate. The oil obtained after the filtrate was concentrated under reduced pressure was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 9:1) to obtain the compound of formula S1 (4.4 g, yield 68%) as a white solid.
[0107] The structural identification of the compound of formula S1 is as follows:
[0108] 1 H NMR (500MHz, CDCl 3 )δ9.82(s,1H),7.44(d,J=2.0Hz,1H),7.39(dd,J=8.3,2.0Hz,1H),6.95(d,J=8.3Hz,1H),6.09- 6.01(m,1H),5.97(br.s,1H),5.42(ddd,J=17.3,2.8,1.6Hz...
Embodiment 2
[0109] Embodiment 2, preparation formula II compound
[0110]
[0111]The compound of formula S1 (534 mg, 3.0 mmol) was dissolved in dichloromethane (15 mL), and imidazole (347 mg, 5.1 mmol) and tert-butyldimethylsilyl chloride (634 mg, 4.2 mmol) were added sequentially at room temperature, and reaction 5 Hours later, add saturated sodium bicarbonate solution to the reaction system, dichloromethane extraction three times, 30 mL each time, combine the organic phases and wash with saturated brine, dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain The oil was subjected to flash silica gel column chromatography (petroleum ether: ethyl acetate = 40:1) to obtain the compound of formula II (867 mg, yield 99%).
[0112] Formula II compound is carried out structural identification as follows:
[0113] 1 H NMR (500MHz, CDCl 3 )δ9.81(s,1H),7.44(dd,J=8.3,2.0Hz,1H),7.37(d,J=2.0Hz,1H),6.95(d,J=8.3Hz,1H),6.11– 6.03(m,1H),5.43(dq,J...
Embodiment 3
[0114] Embodiment 3, preparation formula S2 compound
[0115]
[0116] 3,5-Dihydroxybenzoic acid (5.0 g, 32.4 mmol) was dissolved in N,N-dimethylformamide (100 ml). Cesium carbonate (53.0 g, 162 mmol) was added thereto, and after stirring at room temperature for 5 minutes, allyl bromide (11.2 ml, 129.6 mmol) was slowly added dropwise to the reaction system. Under the protection of nitrogen, the reaction was carried out at room temperature for 12 hours. After the reaction, filter through celite and collect the filtrate. Concentrate under reduced pressure to remove most of N,N-dimethylformamide, dissolve the remaining sample in ethyl acetate (50ml), and wash with water three times, 50mL each time. The organic phase was washed once with saturated brine (50ml). The organic phase was collected and concentrated under reduced pressure to obtain the compound of formula S2 (8.28 g, yield 93%) as light yellow oil.
[0117] The structural identification of the compound of formula ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com