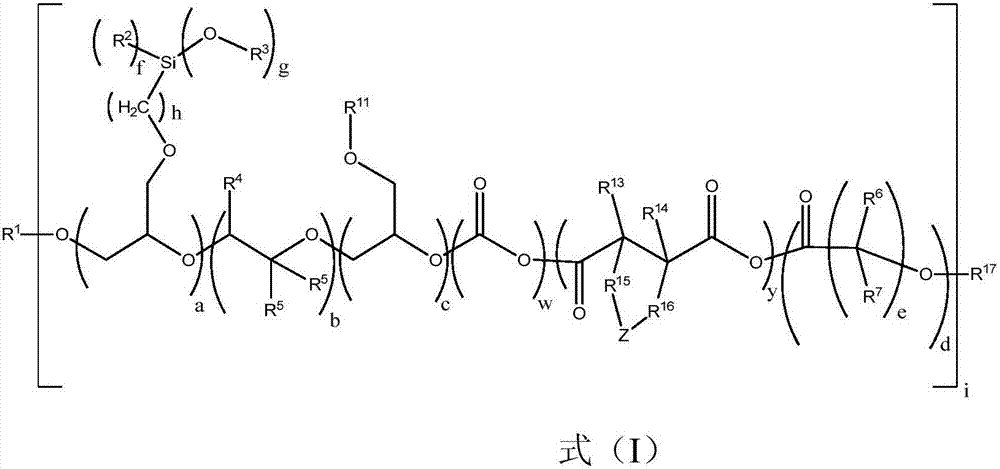
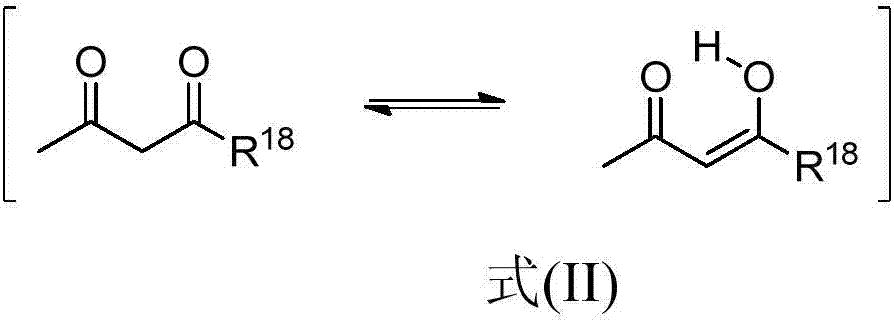
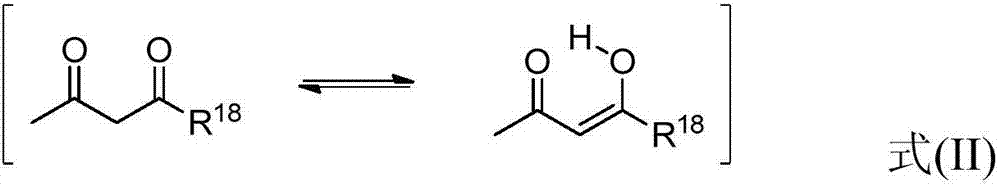
Modified alkoxylation products having at least one non-terminal alkoxy silyl group, with increased storage stability and improved elongation, and polymers produced using said products
A technology of alkoxysilyl and alkoxylation, which is used in the field of adhesives and sealants containing alkoxysilyl, which can solve the problems of deprivation of freedom and achieve improved fluidity and good processability Sexual, high-strength effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238] Embodiment 1 (the present invention):
[0239] Synthesis of PPG-based alkoxysilyl-functional polyethers:
[0240] A 5 liter autoclave was charged with 500 g of PPG 2000 and 150 ppm (based on total batch) of zinc hexacyanocobaltate-double metal cyanide catalyst was added. The reactor was inerted by filling with nitrogen to a pressure of 3 bar and then depressurizing to atmospheric pressure. This operation was repeated two more times. While stirring, the contents of the reactor were heated to 130°C and evacuated to about 20 mbar to remove volatile components. After 30 minutes, the catalyst was activated by metering 60 g of propylene oxide into the evacuated reactor. The internal pressure initially rises to about 0.9 bar. After about 9 minutes, the reaction started, which was seen by the drop in reactor pressure. Then, 1250 g of propylene oxide were metered in continuously over about 55 minutes. This was followed by a further reaction of one hour during which the tem...
Embodiment 2
[0241] Embodiment 2 (the present invention):
[0242] Synthesis of PPG-based alkoxysilyl-functional polyethers:
[0243] A 5 liter autoclave was charged with 333 g of PPG 2000 and 150 ppm (based on total batch) of zinc hexacyanocobaltate-double metal cyanide catalyst was added. The reactor was inerted by filling with nitrogen to a pressure of 3 bar and then depressurizing to atmospheric pressure. This operation was repeated two more times. While stirring, the contents of the reactor were heated to 130°C and evacuated to about 20 mbar to remove volatile components. After 30 minutes, the catalyst was activated by metering 50 g of propylene oxide into the evacuated reactor. The internal pressure initially rises to about 0.9 bar. After about 15 minutes, the reaction started, which was seen by the drop in reactor pressure. Then, 117 g of propylene oxide were metered in continuously over about 5 minutes. Thereafter a further reaction was carried out for 1 hour. A mixture of 5...
Embodiment 3
[0245] Embodiment 3 (comparative embodiment):
[0246] Capping of the polyether from example 1 with isophorone diisocyanate (process according to EP 2636696):
[0247] 750.8 g of the silylpolyether from Example 1 were first charged under nitrogen into a 1 l three-necked flask with a precision glass stirrer and heated to 70°C. Then 33.4 g of IPDI were added, the mixture was stirred for 5 minutes, and 0.05 ml of TIB Kat 216 (dioctyltin dilaurate) was added. The mixture was stirred for 45 minutes, and 67.8 g of the general formula C were added 4 h 9 O[CH 2 CH(CH 3 )O] 5.6 H polyethers. The mixture was then stirred for a further 5 hours at 70°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com