Phthalocyanine pigment composition, coloring composition and color filter
A technology for coloring compositions and phthalocyanine pigments, applied in the field of color filters, can solve the problems of insufficient heat resistance and light resistance, poor dispersibility, foreign matter generation, etc., and achieve the effect of excellent solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
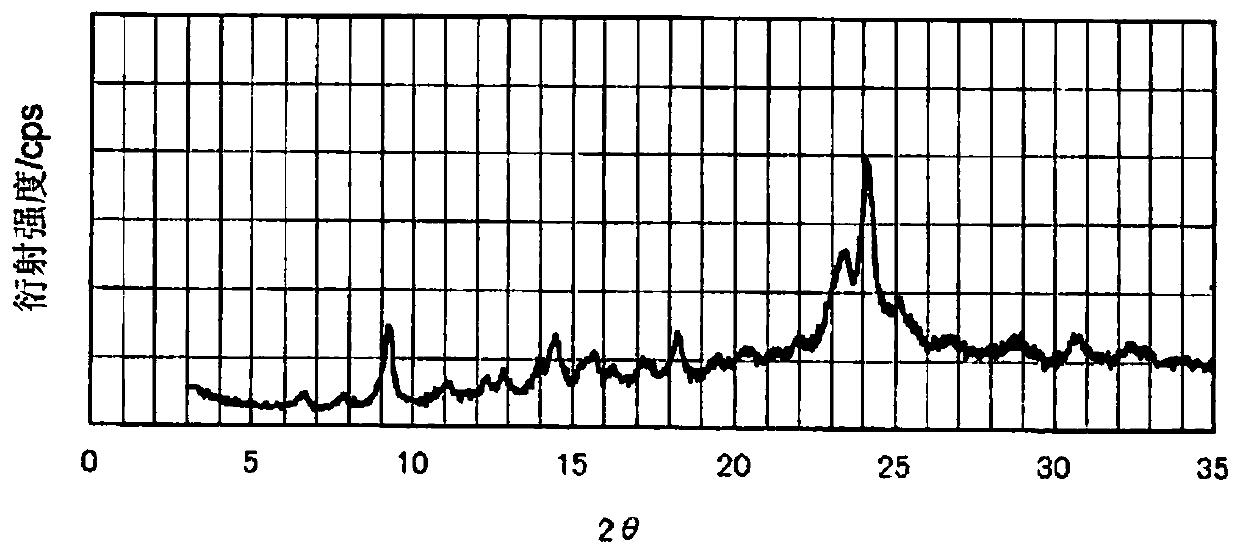
[0270] (Manufacture of Phthalocyanine Pigment (P-1))
[0271] In a reaction vessel, 225 parts of phthalonitrile and 78 parts of aluminum chloride anhydrate were mixed and stirred in 1250 parts of n-amyl alcohol. 266 parts of 1,8-diazabicyclo[5.4.0]undec-7-ene (1,8-Diazabicyclo[5.4.0]undec-7-ene, DBU) were added thereto, and the temperature was raised. Reflux at 136°C for 5 hours. The reaction solution cooled to 30 degreeC in the stirring state was injected into the mixed solvent containing 5000 parts of methanol and 10000 parts of water, stirring, and the blue slurry was obtained. The slurry was filtered, washed with a mixed solvent containing 2000 parts of methanol and 4000 parts of water, and dried to obtain 135 parts of chloroaluminum phthalocyanine (see the following chemical formula (5)).
[0272] As a result of elemental analysis of the obtained chloroaluminum phthalocyanine, the measured values were (C) 66.7%, (H) 3.0% compared to the calculated values (C) 66.85%,...
Embodiment 2
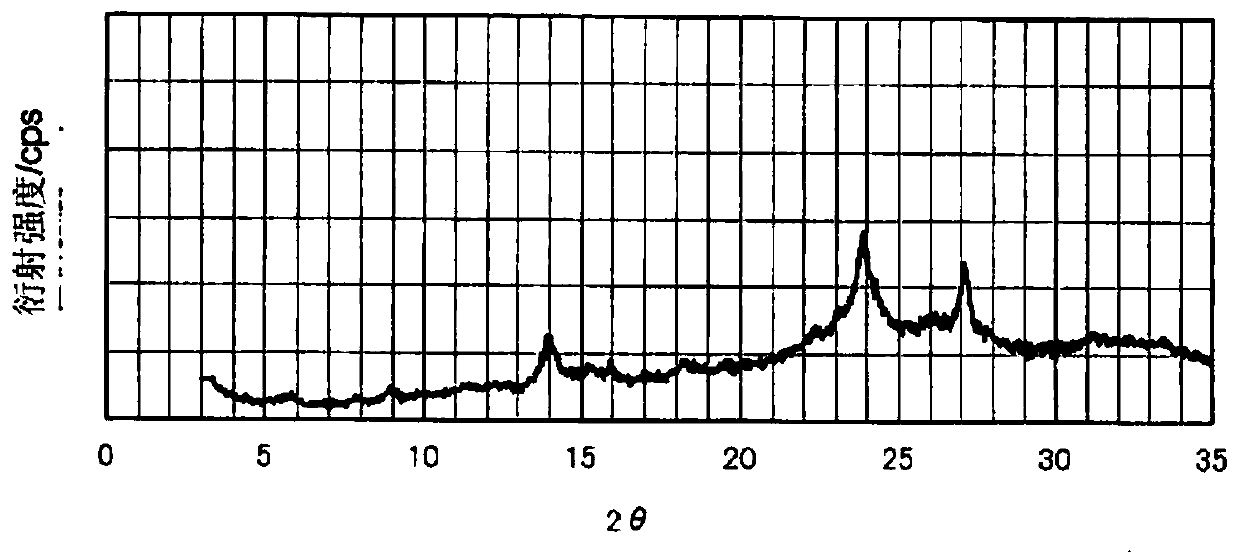
[0277] (Manufacture of Phthalocyanine Pigment (P-2))
[0278] Except that in the manufacture of the phthalocyanine pigment (P-1), 199 parts of 1,3-dibromo-5,5-dimethylhydantoin was changed to 186 parts of N-bromosuccinyl Except for the amine, 143 parts of phthalocyanine pigments (P-2) were produced in the same manner as in Example 1.
[0279] The number of bromine substitutions was calculated for the obtained phthalocyanine pigment (P-2), and it was 6.0 on average, and a peak corresponding to the same molecular weight was confirmed from the mass spectrum, and it was identified as the target compound. In addition, the halogen distribution range is 5. The volume average primary particle diameter of the obtained phthalocyanine pigment (P-2) was 47 nm.
Embodiment 3
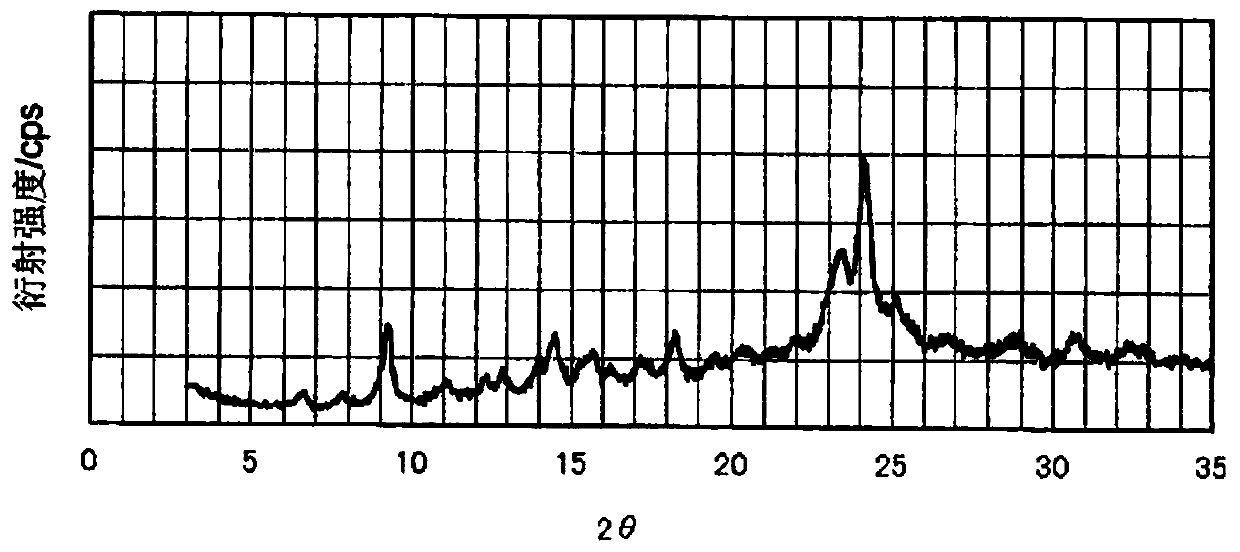
[0281] (Manufacture of phthalocyanine pigment (P-3))
[0282] In addition to changing 199 parts of 1,3-dibromo-5,5-dimethylhydantoin to 249 parts of 1,3-dibromo- Except for 5,5-dimethylhydantoin, 187 parts of phthalocyanine pigments (P-3) were produced in the same manner as in Example 1.
[0283] The number of bromine substitutions was calculated for the obtained phthalocyanine pigment (P-3), and it was 10.3 on average, and a peak corresponding to the same molecular weight was confirmed from the mass spectrum, and it was identified as the target compound. In addition, the halogen distribution range is 7. The volume average primary particle diameter of the obtained phthalocyanine pigment (P-3) was 40 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com