Preparation method of 4-hydroxy ciclosporin
A technology of hydroxycyclosporine and cyclosporine, which is applied in the field of organic synthesis and can solve problems such as difficult industrial production, low yield, and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
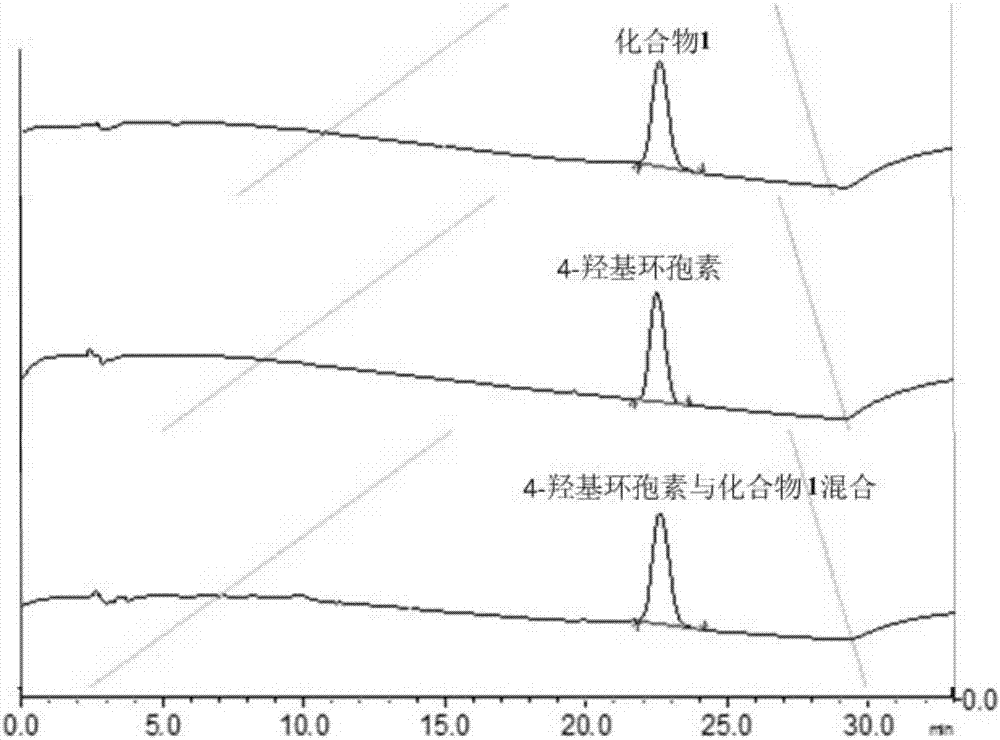
Image
Examples
preparation example Construction
[0049] Concrete, the preparation method of 4-hydroxycyclosporin comprises the following steps:
[0050] Step 1. Dissolve compound 2 and the first reactant in the first organic solvent at -20°C to 35°C (preferably in ice bath), and then add 3- Chloro-2-methylpropene, then heated to 58°C-62°C (preferably 60°C) and fully reacted (reaction time is preferably 2h), then added water and adjusted the pH to 7 with an acidic regulator, purified after sufficient reaction to obtain Compound 3.
[0051] The first reactant is selected from NaH, KH, K 2 CO and Cs 2 CO 3 at least one of the .
[0052] The acid regulator is hydrochloric acid, citric acid, sulfuric acid or fluoboric acid. Preferably, the acid regulator is hydrochloric acid (with a concentration of 0.5 mol / L-12 mol / L).
[0053] The structural formulas of compound 2, 3-chloro-2-methylpropene and compound 3 are as follows,
[0054] Compound 2: Compound 3:
[0055] In step 1, NaH and 3-chloro-2-methylpropene were added ...
Embodiment 1
[0137] Step 1. Compound 2 (diethyl acetylaminomalonate, 217.1 g, 1.0 mol) was dissolved in 2.0 L of DMF, and then NaH (60 wt% dissolved in kerosene, 48.0 g, 1.2 mol) was added. 3-Chloro-2-methylpropene (108.7 g, 117.5 mL, 1.2 mol) was then added under ice cooling, and the addition was completed within 30 min. The temperature was then heated to 60°C and maintained for 2 hours. Water (2.0 L) was then added, and the pH of the solution was adjusted to 7 with 1.0 mol / L HCl. At room temperature, reacted for 12 h, the precipitated white solid was collected by filtration, washed with distilled water (2×1 L), and finally dried in a vacuum oven to obtain compound 3 (247.0 g, 91%).
[0138] Step 2. Dissolve compound 3 (108.6 g, 0.5 mol) in 0.5 L of ethanol, add 0.5 L of 3.0 M NaOH aqueous solution, react under reflux for 3 h, then remove ethanol, and then adjust the pH with concentrated hydrochloric acid (12 mol / L) The value was 7, refluxed for 2h, and then the pH value of the solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com