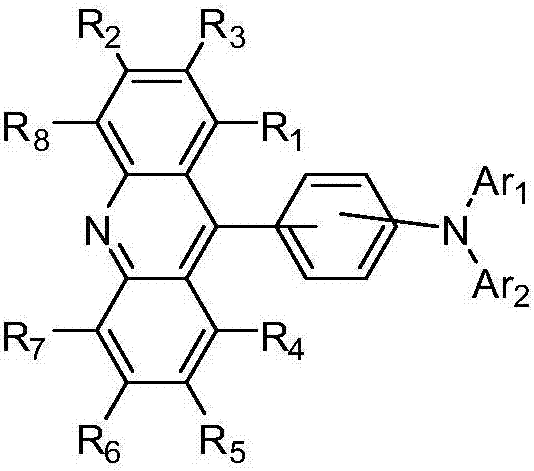
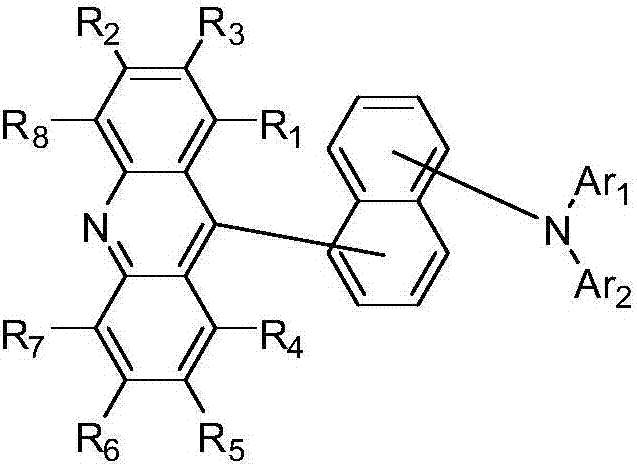
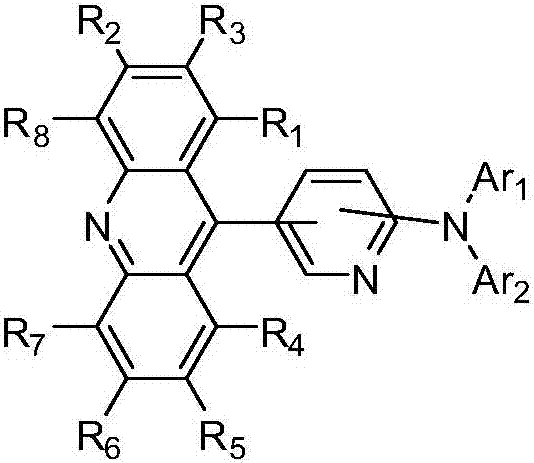
Novel acridine compound and organic light-emitting devices thereof
A compound, nitrogen-anthracene technology, applied in the field of organic optoelectronic materials, can solve the problems of inability to improve and reduce luminous efficiency, and achieve the effects of improving hole mobility, good luminous efficiency, and high brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1. Production of Intermediate C-1
[0084]
[0085] Under the protection of nitrogen, nitrogen anthracene (18.0 g, 100 mmol) was added into the reaction vessel and dissolved with tetrahydrofuran (200 ml). Add N-bromosuccinimide (18.0 g, 101 mmol) at 0°C and stir at room temperature for 5 hours. After the reaction was terminated, the reaction was quenched with distilled water, and the organic matter was extracted with ethyl acetate. After filtering through a silica gel filter funnel, the organic solvent was concentrated and recrystallized with ethyl acetate to obtain 9-bromoazanthracene (23.2 g, 90%).
Embodiment 2
[0086] Embodiment 2. The manufacture of intermediate B-1
[0087]
[0088] 9-Bromoazephanthracene (10g, 38.74mmol) was added to a three-necked flask, THF (100mL) was added, under nitrogen protection, stirred at -78°C for 30 minutes, n-butyllithium (2.5M / 21mL) was added, reacted for 1 hour, and then Add triisopropyl borate (14 g), react at low temperature for 1 hour, and gradually return to room temperature. During post-treatment, add 2M hydrochloric acid to make the pH of the solution 4-5, statically separate the liquids, extract the aqueous layer once with ethyl acetate, combine the organic layers, and spin dry to obtain azanthracene boronic acid (4.85 g, y=55%).
Embodiment 3
[0089] Example 3. Production of Intermediate A-1
[0090]
[0091] Under nitrogen, bis(4-biphenylene)amine (19.0g, 59.1mmol), bisbromobiphenyl (18.44g, 59.1mmol), tris(dibenzylideneacetone)dipalladium (0.1g ), tri-tert-butylphosphine (15%, 0.3g), sodium tert-butoxide (1.8g), toluene (300ml), and stirred at 60°C for 12 hours. After the reaction solution was cooled, it was filtered with a silica gel filter, and after the solution was concentrated, it was subjected to column chromatography with dichloromethane and hexane to obtain (A-1) (22.21 g, yield 68%).
[0092] Intermediates A-2 to A-28 in Table 1 were prepared in the same manner as A-1.
[0093] Table 1: Preparation of intermediates A-2 to A-28
[0094]
[0095]
[0096]
[0097]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com