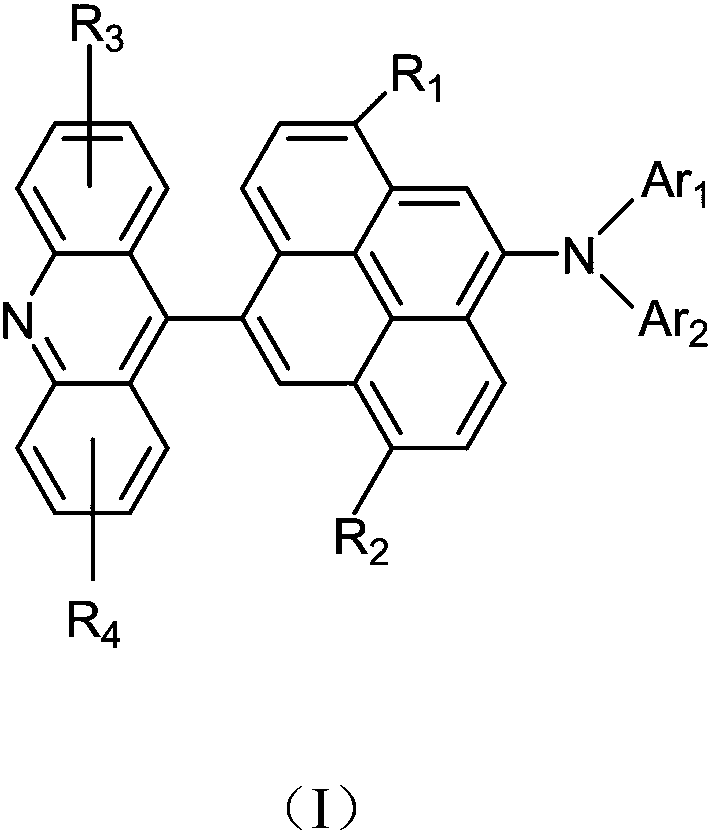
Acridine derivative and organic light emitting device with same
A technology of organic light-emitting devices and derivatives, used in light-emitting materials, organic chemistry, electrical solid devices, etc., to achieve high efficiency, long life, and excellent heat resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound 1
[0042]
[0043]Tri-tert-butylphosphine (4.4 mL of a 1.0 M solution in toluene, 1.48 g, 0.05 mmol), palladium acetate (0.4 g, 1.83 mmol) and sodium tert-butoxide (22.7 g, 237 mmol) were added to 2,7-bis A solution of isopropylacridine (4.82g, 18.3mmol) and 4,9 dibromo-1,6 diisopropylpyrene (8.13g, 18.3mmol) in degassed toluene (500mL), and the mixture was Heat at reflux for 2 hours. The reaction mixture was cooled to room temperature, diluted with toluene and filtered through celite. The filtrate was diluted with water and extracted with toluene, and the combined organic phases were evaporated under vacuum. The residue was filtered through silica gel and recrystallized to obtain Intermediate 1-A (9.17 g, 80% of theory).
[0044] Mass Spectrum m / z: 625.24 (calculated: 625.23). Theoretical element content (%)C 41 h 40 BrN: C, 78.58; H, 6.43; Br, 12.75; N, 2.24 Measured element content (%): C, 78.58; H, 6.43; Br, 12.76;...
Embodiment 2
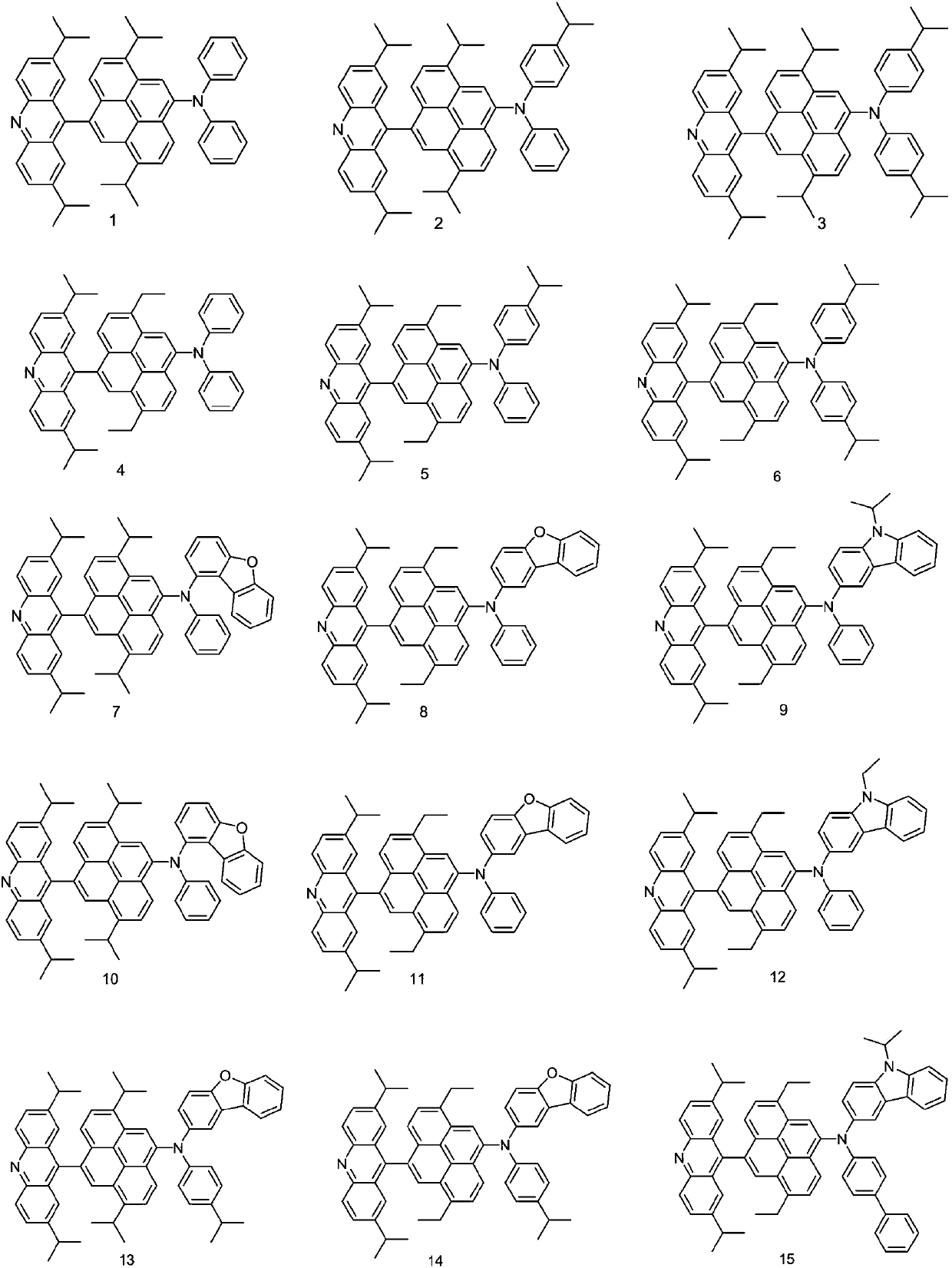
[0049] Embodiment 2: the preparation of compound 7
[0050] The synthesis steps of intermediate 7-A are the same as those of 1-A in Example 1.
[0051] Preparation of Compound 7:
[0052]
[0053] Add 7-a (15.32g, 59.1mmol), 7-A (37.03g, 59.1mmol), tris(dibenzylideneacetone) dipalladium (0.67g, 0.58mmol), tri-tert-butyl Phosphine (15%, 0.15g), sodium tert-butoxide (0.9g), toluene (400ml), stirred at 60°C for 12 hours. After the reaction solution was cooled, it was filtered with a silica gel filter, and after the solution was concentrated, it was subjected to column chromatography with dichloromethane and hexane to obtain compound 7 (38.06 g, 80%).
[0054] Mass Spectrum m / z: 804.42 (calculated: 804.41). Theoretical element content (%)C 59 h 52 N 2 O: C, 88.02; H, 6.51; N, 3.48; O, 1.99 Measured element content (%): C, 88.01; H, 6.52; N, 3.49; O, 1.98. The above results confirmed that the obtained product was the target product.
Embodiment 3
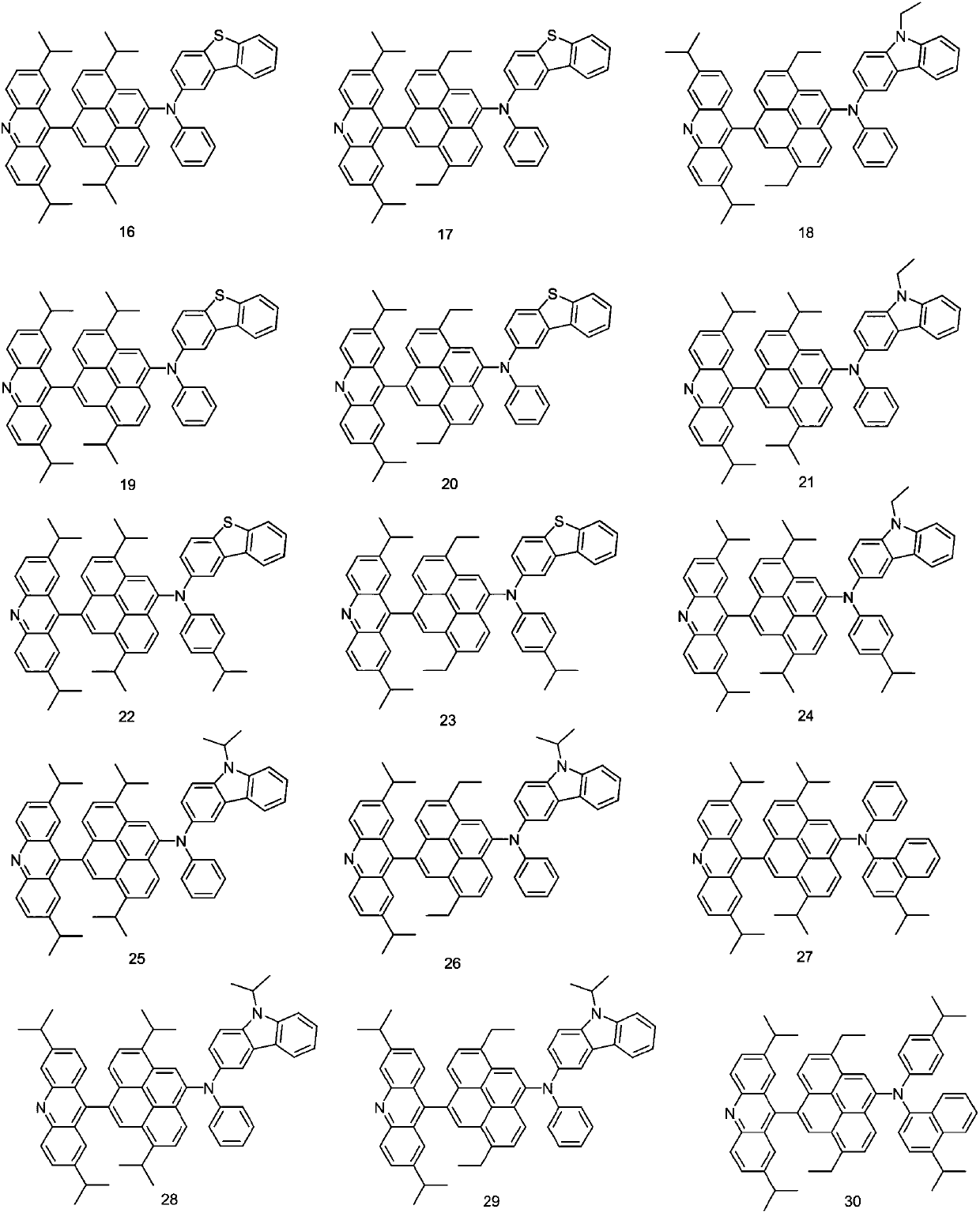
[0055] Embodiment 3: the preparation of compound 16
[0056] The synthesis steps of intermediate 16-A are the same as those of 1-A in Example 1.
[0057] Preparation of compound 16:
[0058]
[0059] Add 16-a (16.27g, 59.1mmol), 16-A (37.03g, 59.1mmol), tris(dibenzylideneacetone) dipalladium (0.67g, 0.58mmol), tri-tert-butyl Phosphine (15%, 0.15g), sodium tert-butoxide (0.9g), toluene (400ml), stirred at 60°C for 12 hours. After the reaction solution was cooled, it was filtered with a silica gel filter, and after the solution was concentrated, it was subjected to column chromatography with dichloromethane and hexane to obtain compound 16 (38.82 g, 80%).
[0060] Mass Spectrum m / z: 820.40 (calculated: 820.39). Theoretical element content (%)C 59 h 52 N 2 S: C, 86.30; H, 6.38; N, 3.41; S, 3.90 Measured element content (%): C, 86.31; H, 6.37; N, 3.40; S, 3.91. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com