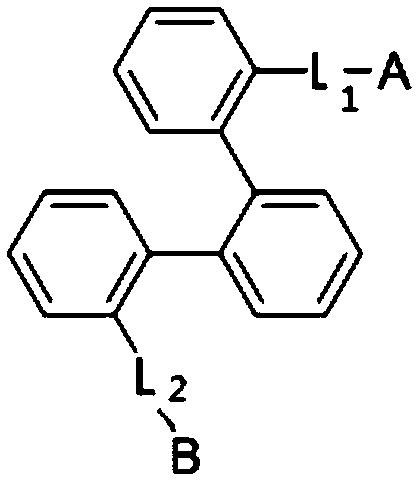
Novel compound and organic light-emitting device including the same
A technology of organic light-emitting devices and compounds, which is applied in the field of novel compounds and organic light-emitting devices containing them, can solve the problems of high driving voltage, short life, low efficiency, etc., and achieve increased hole mobility, prevention of recrystallization, high efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0124] Preparation Example 1: Synthesis of main core (main core) 1
[0125] In order to synthesize the target compound, main core 1 (main core 1) was synthesized as follows.
[0126]
[0127] In a round bottom flask, 100.0 g (603.32 mmol, 1 eq) of 1,2-phenylene diboronic acid (1,2-phenylenediboronic acid), 1-bromo-2-iodobenzene (1-bromo-2-iodobenzene) 341.3g (1206.64mmol, 2eq), Pd(PPh 3 ) 4 34.85g (30.17mmol, 0.05eq), K 3 PO 4 256.13g (1206.64mmol, 2eq) was dissolved in 1,4-dioxane (1,4-dioxan) 1000ml, H 2 After O 200mL, reflux and stir. After 2 hours, completion of the reaction was confirmed by thin layer chromatography (TLC), and the organic layer was extracted with methylcellulose (MC). After the extracted substance was filtered under reduced pressure, it was recrystallized from MC and n-hexane (Hexane) to obtain 220 g of main core 1 (yield = 94.0%, white solid (White solid)).
preparation example 2
[0128] Preparation Example 2: Synthesis of main core (main core) 2
[0129]
[0130] In order to synthesize the target compound, using the main core 1 obtained in the above Preparation Example 1 as a starting material, various reagents (Reagent) (dibenzo[b,p]furan-4-ylboronic acid (dibenzo[b,d ]furan-4-ylboronic acid), dibenzo[b,p]furan-3-ylboronic acid (dibenzo[b,d]furan-3-ylboronic acid), dibenzo[b,p]furan-2- Base boronic acid (dibenzo[b,d]furan-2-ylboronic acid) was used to synthesize main core 2 by Suzuki reaction.
[0131] In a round bottom flask, the main core 1 50.0g (128.8mmol, 1eq), dibenzo [b, p] furan-4-yl boronic acid (dibenzo [b, d] furan-4-ylboronic acid) 24.6g ( 115.94mmol, 0.9eq), Pd(PPh 3 ) 4 7.44g (6.44mmol, 0.05eq), K 3 PO 4 54.7g (257.6mmol, 2eq) was dissolved in 1,4-dioxane (1,4-dioxane) 500mL, H 2 After O 100mL, reflux and stir. After 1 hour, completion of the reaction was confirmed by thin layer chromatography (TLC), and the organic layer wa...
Embodiment 1
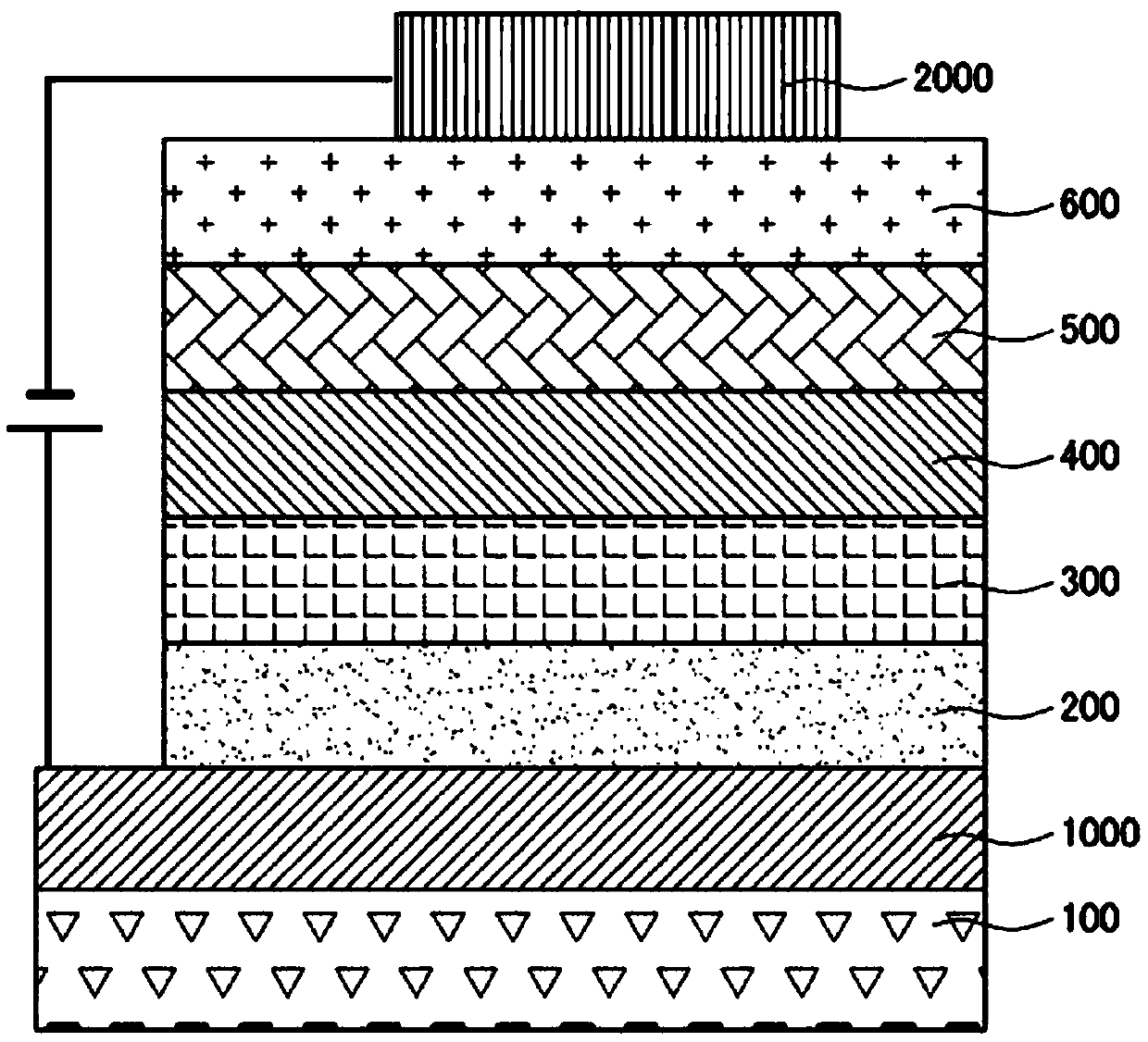
[0172] Example 1: Use as a luminescent auxiliary layer (second hole transport layer)
[0173] ultrasonication of distilled water The thickness of the glass substrate coated with indium tin oxide (ITO) as a thin film is washed. When the distilled water washing is completed, use a solvent such as isopropanol, acetone, methanol, etc. to perform ultrasonic cleaning, and after drying, transfer to a plasma cleaner, and then use oxygen plasma to clean the above-mentioned substrate for 5 minutes. The upper part of the substrate uses a thermal vacuum depositor (thermal evaporator) as a hole injection layer to DNTPD, HATCN for film formation, as the first hole transport layer will NPB for film formation, as the second hole transport layer will After compound 1 was formed into a film, as the above-mentioned light-emitting layer doped with 3% of BH01:BD01, to Make a film. Next, as the electron transport layer will After the ET01:Liq (1:1) was used to make the film, the of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com