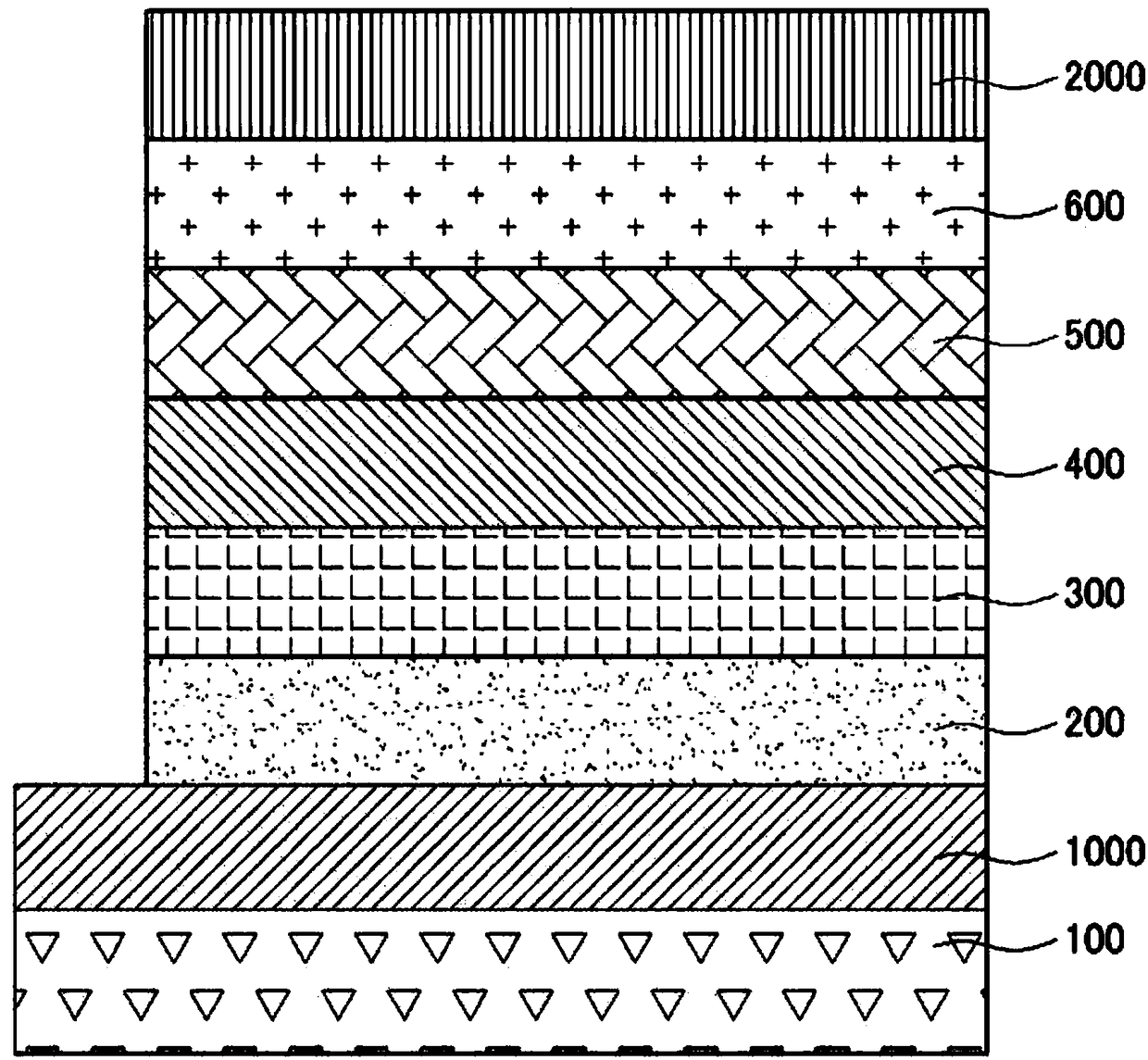
Novel compound and organic electroluminescent device including the same
A technology of organic light-emitting devices and compounds, applied in the field of novel compounds and organic electroluminescent devices containing them, can solve the problems of high driving voltage, low efficiency, short life, etc., achieve high color purity, high luminous efficiency, and prolong life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
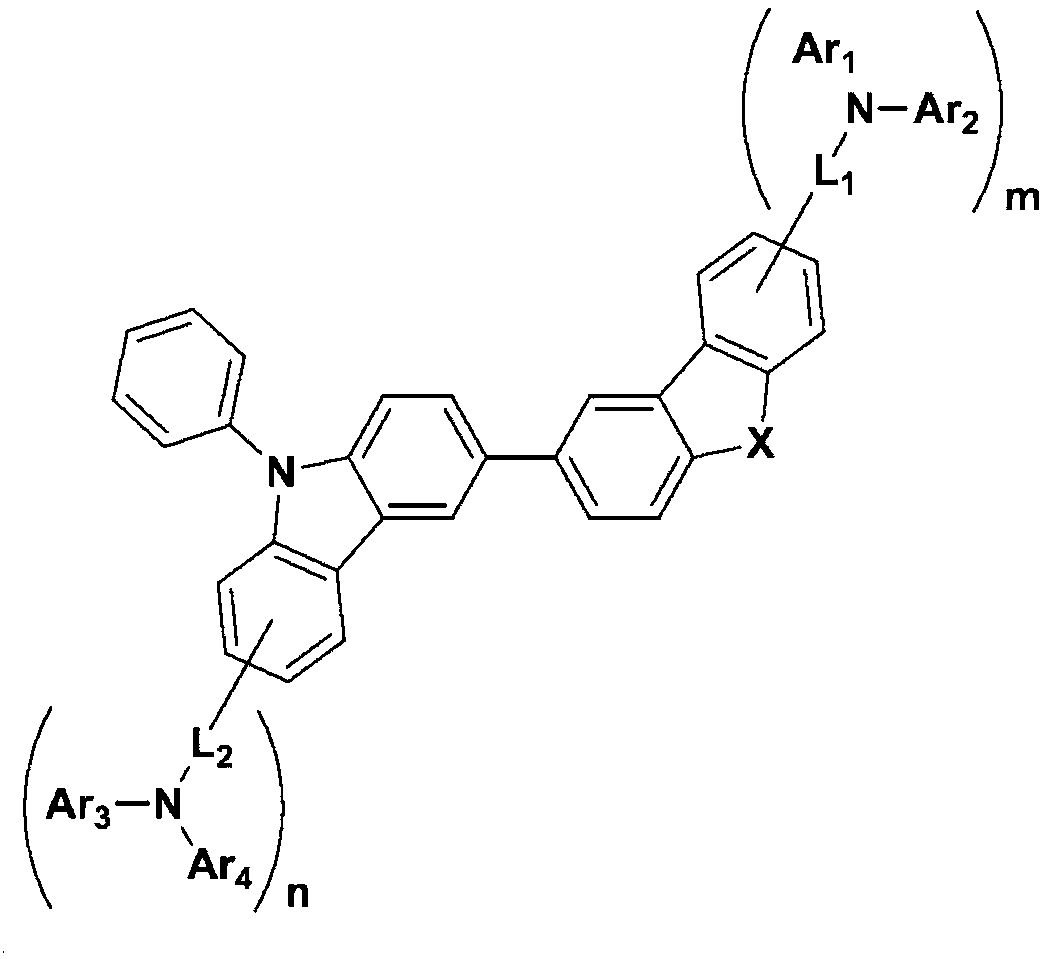
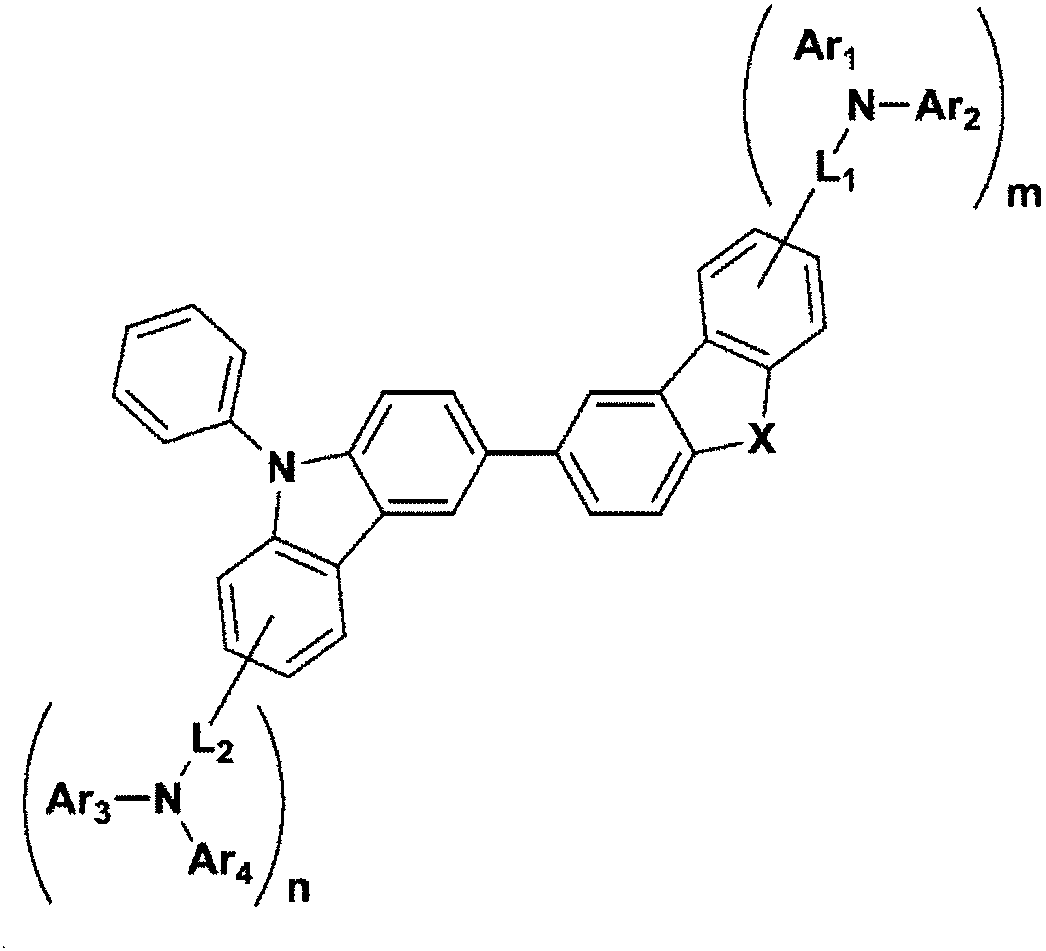
[0035] The first embodiment of the present invention provides a compound represented by the following chemical formula 1:
[0036] chemical formula 1
[0037]
[0038] In the above chemical formula, X is O or S, L 1 and L 2 are independently directly coupled, substituted or unsubstituted C 6 -C 30 Arylene or substituted or unsubstituted C 3 -C 30 Heteroarylene, Ar 1 to Ar 4 are independently substituted or unsubstituted C 6 -C 30 Aryl or substituted or unsubstituted C 3 -C 30 In the heteroaryl group, m and n are each independently 0 or 1, and the sum of m and n is 1 or more.
[0039] In an example of the present invention, in the compound of the above chemical formula 1, the third position of carbazole is fixed at the second position of the dibenzofuran or dibenzothiophene structure, and in the case of changing this binding position, For example, in the case of combining with the fourth position of the dibenzofuran or dibenzothiophene structure, relatively low T...
Synthetic example 1
[0179] Synthesis Example 1: Synthesis of Intermediate (I-1)
[0180]
[0181] Under argon or nitrogen atmosphere, in 65.2g (200mM) of 2,8-dibromodibenzo[b,d]furan, 60.3g (210mM) of (9-phenyl-9H-carbazol-3-yl ) boric acid, 4.7g (4mM) tetrakis (triphenylphosphine) palladium (0), add 600ml of toluene, concentration of 2M Na 2 CO 3 300ml of aqueous solution was heated under reflux for 15 hours. After the reaction is over, use dichloromethane (dichloromethane) to extract, and put MgSO 4 to filter. After removing the solvent of the filtered organic layer, purification was performed by column chromatography to obtain 58.7 g of intermediate I-1 (3-(8-bromodibenzo[b,d]furan-2-yl)-9- phenyl-9H-carbazole) (yield 61%).
[0182] m / z: 487.06 (100.0%), 489.06 (96.4%), 488.06 (33.2%), 490.06 (32.1%), 491.06 (4.7%), 489.06 (2.3%), 489.06 (1.4%), 489.06 (0.8%) )
Synthetic example 2
[0183] Synthesis Example 2: Synthesis of Intermediate (I-2)
[0184]
[0185] Under argon or nitrogen atmosphere, in 84.0g (200mM) of 2,8-diiododibenzo[b,d]furan, 60.3g (210mM) of (9-phenyl-9H-carbazol-3-yl ) boric acid, 4.7g (4mM) tetrakis (triphenylphosphine) palladium (0), add 600ml of toluene, concentration of 2M Na 2 CO 3 300ml of aqueous solution was heated under reflux for 10 hours. After the reaction, use dichloromethane to extract, and put MgSO 4 to filter. After removing the solvent of the filtered organic layer, it was purified by column chromatography to obtain 42.8 g of intermediate 1-2(3-(8-iodobenzo[b,d]furan-2-yl)-9-benzene base-9H-carbazole) (yield 39%).
[0186] m / z: 535.04 (100.0%), 536.05 (31.7%), 537.05 (2.3%), 537.05 (1.9%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com