Genetically engineered bacterium for expressing sucrose phosphorylase and application of genetically engineered bacterium
A technology of sucrose phosphorylase and genetically engineered bacteria, which is applied in the fields of genetic engineering and enzyme engineering, and can solve the problems of unstable enzyme properties, low substrate concentration, unsuitable amylosucrase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Optimization of the gene encoding Leuconostoc mesenteroides sucrose phosphorylase and the construction of the expression vector
[0036] (1) Acquisition of the gene: analyze and optimize the natural SPase gene (1473bp) derived from L. mesenteroides, obtain the gene shown in SEQ ID NO.1, and add two restriction sites (Nde I and Hind III) to the The two ends of the optimized sucrose phosphorylase were synthesized by Shanghai Jierui Bioengineering Co., Ltd. to obtain pGE-sp.
[0037] (2) Construction of expression vectors: the expression vectors pET-24a(+) and pGE-sp were digested with Nde I and Hind III respectively, and ligated with T4ligase. The ligation product was transformed into the expression host Escherichia coli BL21 (DE3) competent cells, and the transformation product was spread on a surface supplemented with 30 μg·mL -1 Grow on Kan's LB plate; Inoculate the normal growth bacteria on the plate to inactivated and add 30μg·mL -1 In Kan's liquid LB med...
Embodiment 2
[0038] Preparation and enzyme activity assay of embodiment 2 sucrose phosphorylase
[0039] (1) Preparation of sucrose phosphorylase
[0040]Inoculate the recombinant bacteria constructed in Example 1 to a concentration of 30 μg·mL -1 In Kan's LB liquid medium, transfer to TB medium (30 μg·mL -1 Kan), after culturing in a shaker at 37°C for 2 hours, IPTG with a final concentration of 0.2mmolL-1 was added to the fermentation broth and the temperature was adjusted to 25°C to induce enzyme production. The fermentation broth was centrifuged (4°C, 8000r / min, 15min) to precipitate the bacteria with 20mM pH7.0Na 2 HPO 4 -NaH 2 PO 4 The supernatant obtained by breaking the wall and centrifuging after buffer reconstitution was the crude enzyme solution.
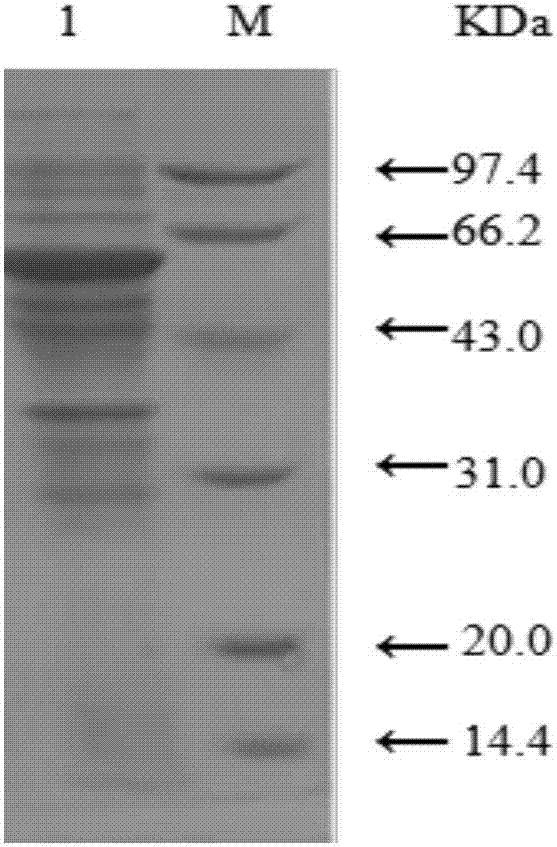
[0041] from figure 1 It can be found that there is a clear band at 53KDa which is consistent with the size of sucrose phosphorylase, indicating that an optimized sucrose phosphorylase can be obtained.
[0042] (2) Enzyme activ...
Embodiment 3
[0045] The condition research of embodiment 3SPase synthesis α-arbutin
[0046] (1) Effect of pH on the synthesis of α-arbutin by SPase
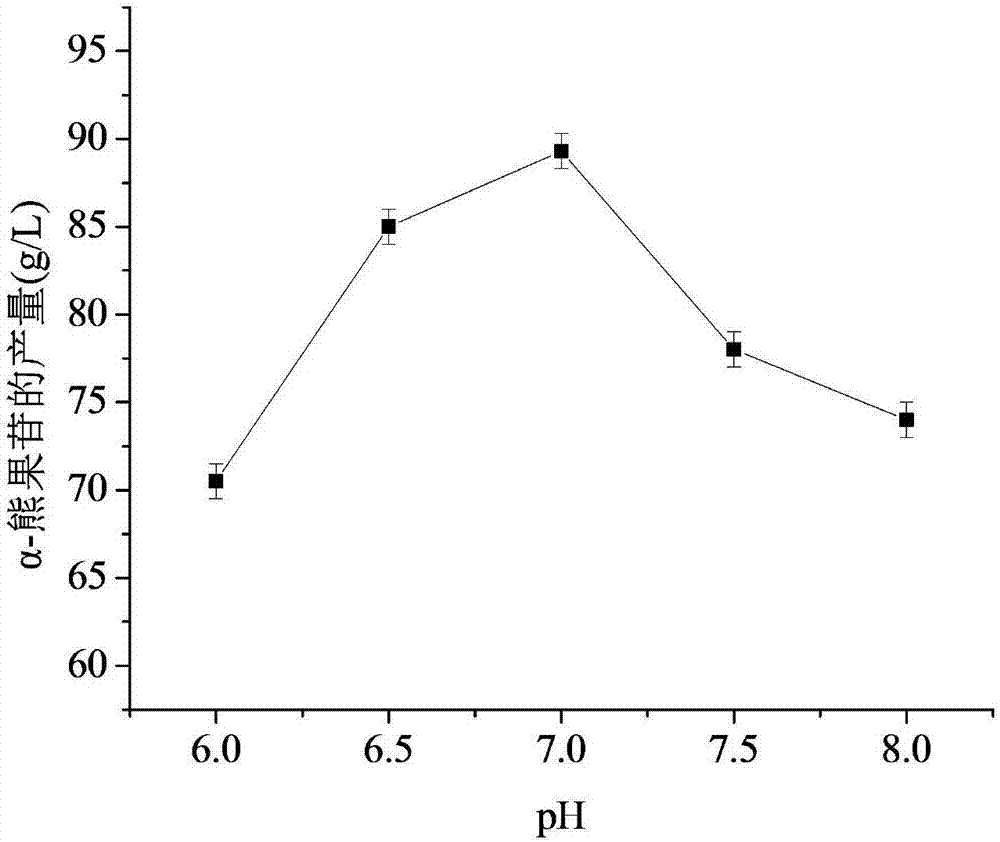
[0047] Respectively carry out the reaction in the range of initial pH 6.0-8.0, other conditions remain unchanged. The result is given by figure 2 Can get, when pH is 7, hydroquinone (HQ) in the reaction has participated in enzymatic transformation and generates product α-arbutin, and the mass concentration of product is 89gL -1 ; When the pH continued to increase, the conversion rate of HQ began to decrease, and the closer to pH 7.0, the higher the yield of α-arbutin.
[0048] (2) Effect of reaction temperature on the synthesis of α-arbutin by SPase
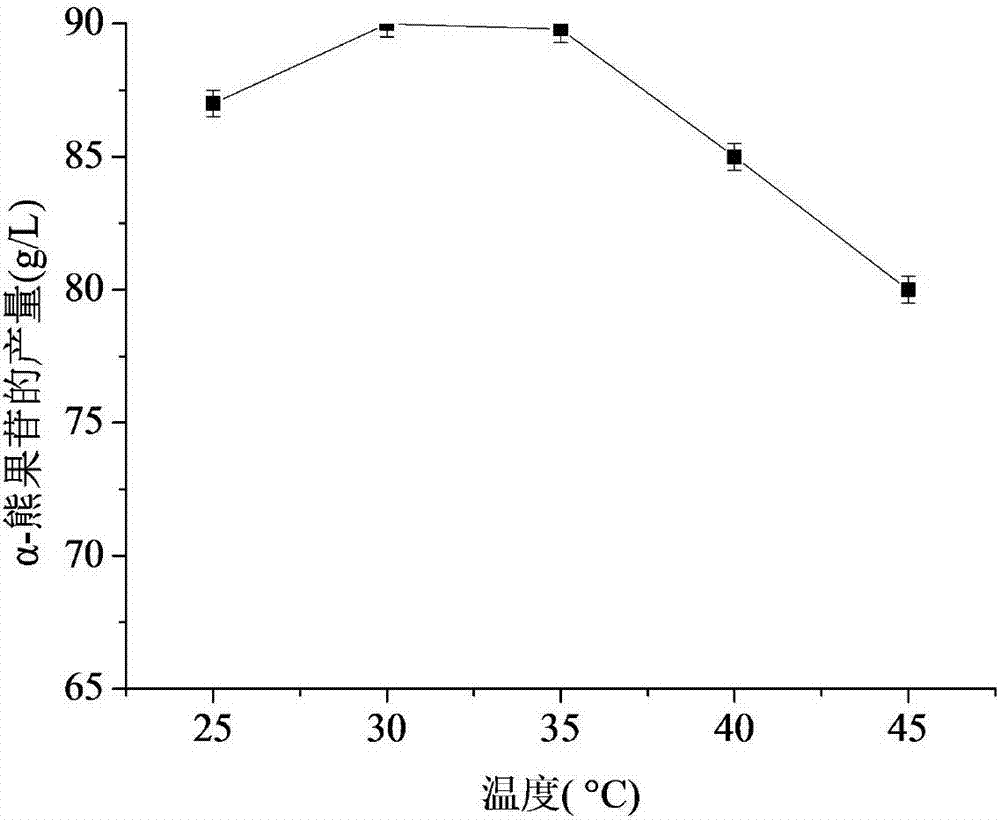
[0049] Carry out the enzyme conversion reaction in the range of 25-45 ℃ respectively, keeping other conditions unchanged, the results are as follows image 3 As shown, as the temperature increases, the yield of α-arbutin increases, and the maximum conversion rate is obtained at 30-35°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com