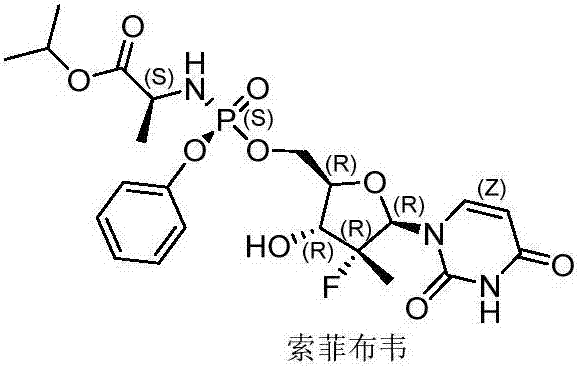
Method for preparing sofosbuvir
A technology for sofosbuvir and a compound, which is applied in the field of drug synthesis and achieves the effects of less by-product content, simple operation and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
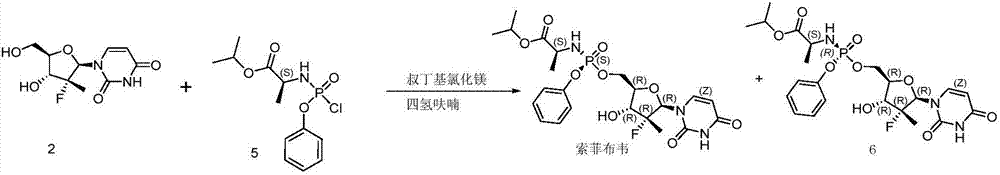
Embodiment 1
[0033] Add 15.0 g of compound 2, 180 g of tetrahydrofuran, and 831 mg of water into the reaction flask, and cool down to -5°C. 71.2 mL of a 1.7M tert-butylmagnesium chloride solution was added, and a solution of 33.9 g of compound 3 in tetrahydrofuran (150 g) was added dropwise. Then react at 5°C, stop the reaction when HPLC detects that the content of sofosbuvir no longer increases. At this time, the HPLC data related to compound 2 in the reaction solution:
[0034] Compound 2(%)
[0035] Add 2mol.L to the reaction solution -1 60g of dilute hydrochloric acid quenched the reaction, extracted with 300g ethyl acetate, and separated the water phase; the organic phase was washed three times with 23g of 8% sodium carbonate solution, combined with the aqueous sodium carbonate phase, added 75g of ethyl acetate for extraction, combined with the organic phase, 45g Brine was washed and evaporated to dryness under reduced pressure. 150g of dichloromethane was added, and the c...
Embodiment 2
[0037] Add 10.0 g of compound 2, 100 g of tetrahydrofuran, and 761 mg of water into the reaction flask, and cool down to -5°C. 47.8 mL of a 1.7 M tert-butylmagnesium chloride solution was added, and a solution of 22.6 g of compound 3 in tetrahydrofuran (100 g) was added dropwise. Then react at 5°C, stop the reaction when HPLC detects that the content of sofosbuvir no longer increases. At this time, the HPLC data related to compound 2 in the reaction solution:
[0038] Compound 2(%)
[0039] Add 2mol.L to the reaction solution -1 40g of dilute hydrochloric acid quenched the reaction, extracted with 200g ethyl acetate, and separated the water phase; the organic phase was washed three times with 15g of 8% sodium carbonate solution, combined with the aqueous sodium carbonate phase, added 50g of ethyl acetate for extraction, combined with the organic phase, 30g Brine was washed and evaporated to dryness under reduced pressure. 100g of dichloromethane was added, and the ...
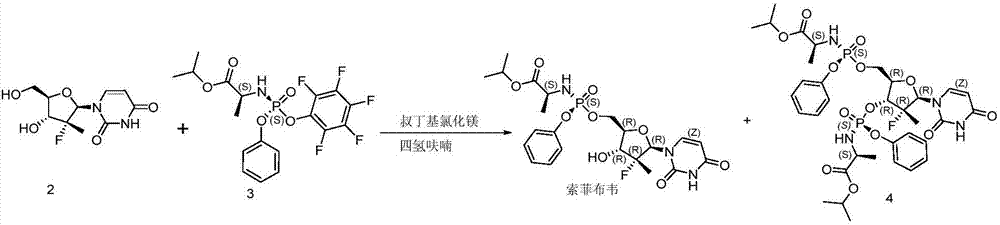
Embodiment 3
[0041] Preparation of Sofosbuvir with different equivalents of water:
[0042] Add 10 g of compound 2, 100 g of tetrahydrofuran, and corresponding equivalents of water into the reaction flask respectively. Cool down to -5°C, add 2.1 equivalents of tert-butylmagnesium chloride solution, then dropwise add 22.6g of compound 3 in tetrahydrofuran (100g) solution, react at 5°C, and stop the reaction when HPLC detects that the content of sofosbuvir no longer increases.
[0043]
[0044]
[0045] According to the experimental results, it can be known that when the end of the reaction is reached, the composition of compound 2, sofosbuvir, and compound 4 in the reaction solution changes with the equivalent number of water added. When using tert-butylmagnesium chloride without adding water, the reaction not only cannot be complete, but also has poor selectivity, and there are more excessively acylated by-products 4, which is consistent with literature reports. With the addition of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com