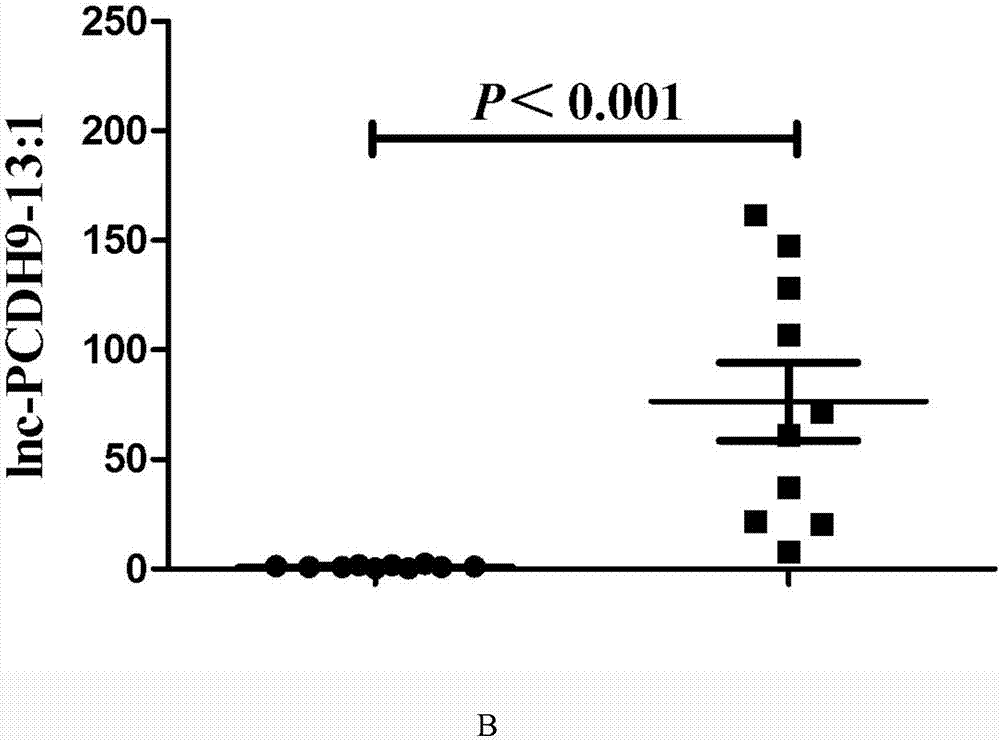
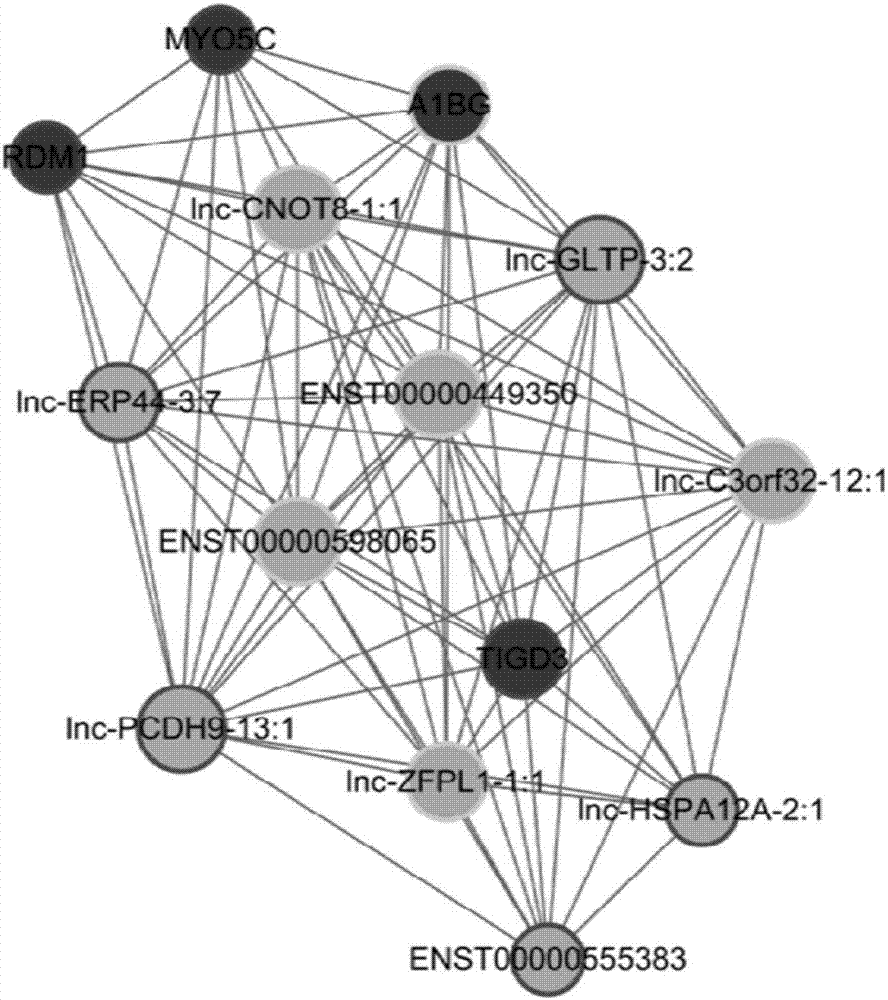
Application of lnc-PCDH9-13:1 detection reagent to preparation of liver cancer diagnosis reagent/kit
A technology of detection reagents and kits, which is applied in the determination/inspection of microorganisms, DNA/RNA fragments, specific-purpose bioreactors/fermenters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Saliva Collection
[0056] Before the saliva collection, the study individuals were required to fast for more than 2 hours from eating, drinking, smoking and oral cleaning.
[0057] In order to increase the amount of saliva collected, use 2% citric acid solution to moisten the cotton tip of a sterile cotton swab, and then put the cotton tip on the rear side wall of one side of the individual's tongue for about 5 seconds, spit out the saliva, and then put the cotton swab on the other side The posterior wall of the tongue. so repeatedly collected. The amount of saliva collected should be more than 5ml, and the collection tube should be a 50ml sterile enzyme-free centrifuge tube. Centrifuge at 4°C, 3000g for 15min, take the supernatant from the supernatant, transfer it to a 1.5ml Eppendoff tube, then centrifuge at 4°C, 12,000g for 10min, then discard the precipitate and take the supernatant. All specimens were stored at -80°C after processing.
Embodiment 2
[0058] Example 2 Extraction of saliva total RNA
[0059] 1. Divide 1ml of saliva evenly on two 1.5ml enzyme-free EP tubes, that is, each EP tube contains 500μl of saliva, then add 15μl of proteinase K (20mg / ml) to each EP tube, mix well , and incubated overnight in a 65°C water bath. Because saliva contains more proteins such as digestive enzymes, which affects the effect of subsequent extraction of salivary RNA, this method can remove the influence of salivary proteins on the experiment.
[0060] 2. Add 0.5ml of acid-phenol-chloroform mixture (the main components are hydrochloric acid, phenol and chloroform) to each of the two EP tubes into the above-mentioned saliva mixture.
[0061] Configuration of acid phenol chloroform
[0062] 2.1 The volume ratio of phenol to chloroform is 5:1
[0063] 2.2 Use concentrated hydrochloric acid to adjust the pH value, and set the pH value at about 4.5, which is most conducive to the extraction of RNA. Mix well.
[0064] 3. Shake by ha...
Embodiment 3
[0076] Example 3 Reverse transcription and expression detection of salivary lnc-PCDH9-13:1
[0077] The expression level of lnc-PCDH9-13:1 in saliva was detected by qPCR instrument, and expressed relatively quantitatively, wherein the internal reference was β-actin.
[0078] 1. Reverse transcription reaction of salivary lnc-PCDH9-13:1
[0079] Take 2 μL of RNA for reverse transcription. After reacting at 65°C for 5 minutes, immediately ice-bath for 2 minutes.
[0080]Add 2 μl 5×RT Buffer, 0.5 μl Enzyme Mix, 0.5 μl PrimerMix (both products of Toyobo Company, Cat. No. FSK-100) and 5 μl nuclease-free water treated with DEPC to the above 2 μL RNA, and then continue reverse transcription, The reaction conditions were 37°C for 15 minutes, 98°C for 5 minutes, and 4°C∞.
[0081] 2. Detection of expression level of saliva lnc-PCDH9-13:1 (qPCR reaction)
[0082] Take 3 μL of the reverse transcription product as a template, and use SYBR Premix Ex TaqⅡ kit (TaKaRa Company, Cat. downs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com