SNP marker associated with breast cancer chemotherapy toxicity and application of SNP marker
A technique for chemotherapy toxicity, breast cancer, applied in the field of SNP markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This embodiment is the collection of samples and the arrangement of sample data.
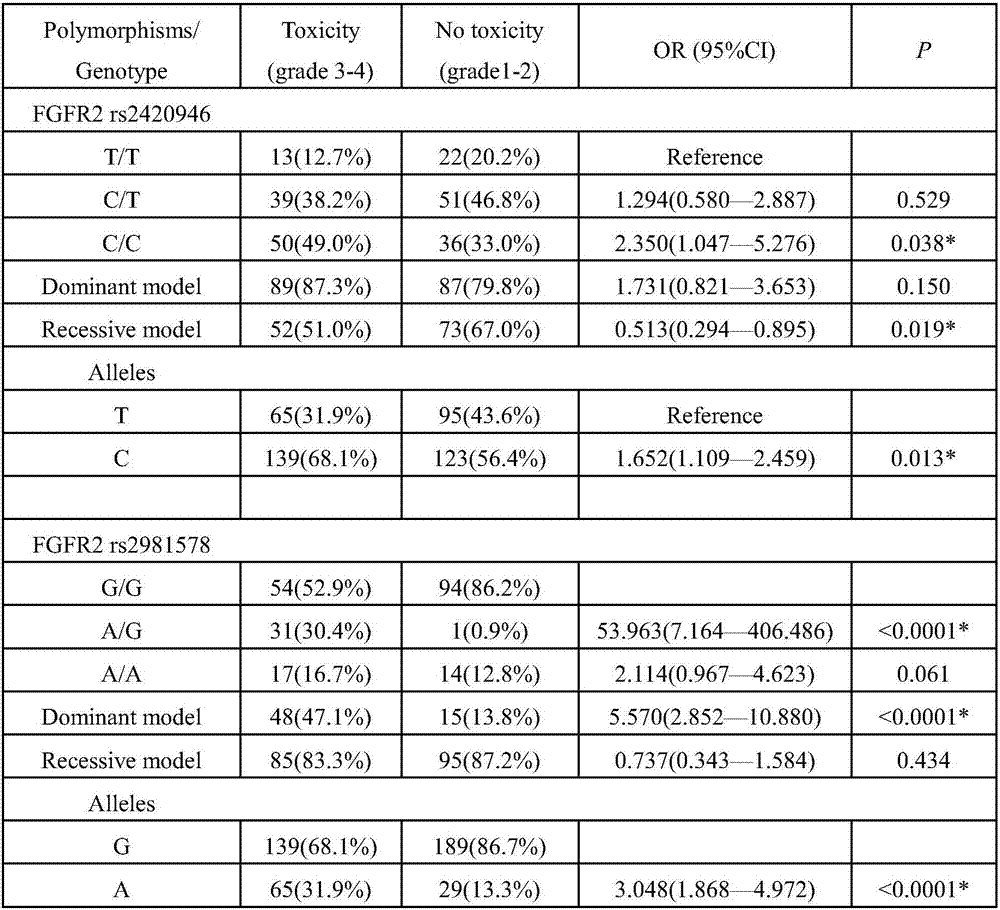
[0048] From September 2015 to November 2016, the inventor collected a large number of blood samples from breast cancer chemotherapy patients in Huashan Hospital (North Hospital) affiliated to Fudan University. After sorting out the sample data, the inventor selected 211 cases that met the following criteria: Standard samples, the sample selection criteria are as follows: (1) New-onset breast cancer patients who were admitted to the hospital for the first time and confirmed by histopathology or cytology, and their diagnosis was confirmed by at least two pathologists according to the standards issued by the World Health Organization; ( 2) The blood routine and liver and kidney function tests were normal before the first chemotherapy; (3) Received 4 cycles of cyclophosphamide + docetaxel + epirubicin chemotherapy; radiotherapy before or during chemotherapy was excluded (4) Have detailed bloo...
Embodiment 2
[0050] This embodiment is the peripheral blood DNA extraction, genotype detection and result analysis.
[0051] The specific steps are:
[0052] 1) Process the blood material, take 200 μl of blood sample.
[0053] 2) Add 20 μl Proteinase K solution and mix well.
[0054] 3) Add 200 μl of buffer solution GB, fully invert and mix, and place at 56°C for 10 minutes, during which invert and mix several times, the solution should become clear.
[0055] 4) Add 200 μl of absolute ethanol and mix thoroughly by inversion, at this time flocculent precipitation may appear.
[0056] 5) Add the solution and flocculent precipitate obtained in the previous step into an adsorption column CB3 (the adsorption column CB3 is placed in a collection tube), centrifuge at 12000rpm (~13400×g) for 30sec, pour off the waste liquid in the collection tube, and put the adsorption Column CB3 was placed in a collection tube.
[0057] 6) Add 500μl buffer GD to the adsorption column CB3 (check whether absol...
Embodiment 3
[0068] This embodiment is the production of a kit for detecting or predicting chemotherapy toxicity of breast cancer.
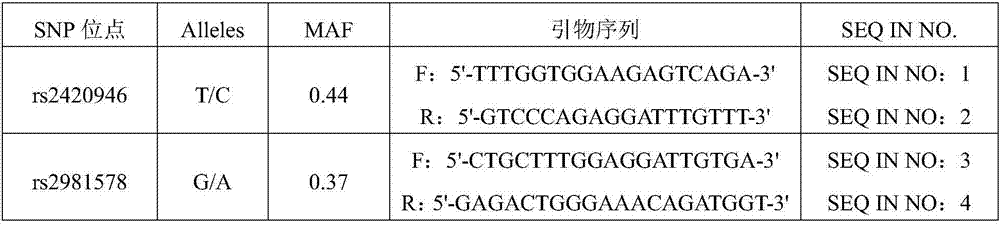
[0069] The production and operation process of the SNP kit is based on the detection technology of the Sequenom Mass ARRAY genotyping platform. The kit contains a batch of SNP-specific amplification primers (including the following primers: the rs2420946 amplification primer whose sequence is SEQ ID No:1 and SEQ ID No:2 and / or whose sequence is SEQ ID No:3 and SEQ ID No:4 rs2981578, see Table 1 for details), and common reagents required for corresponding PCR techniques, such as: dNTPs, MgCl 2 , double distilled water, etc., these commonly used reagents are well known to those skilled in the art, and there may also be standards and controls (such as standards for genotype determination and blank controls, etc.). The value of this kit is that it only needs peripheral blood and no other tissue samples, detects SNP through the most streamlined and specific ampli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com