Oxidation-responsive PEGlated lipid material, and preparation method and application thereof
A technology of lipid materials and click chemical reactions, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, emulsion delivery, etc., and can solve problems that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
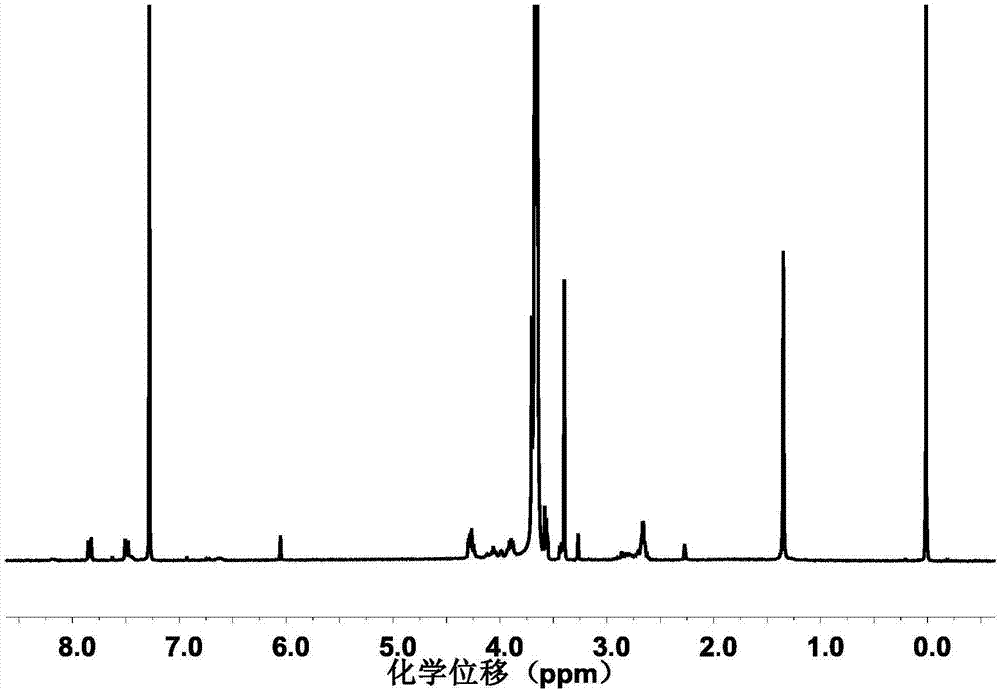
Image
Examples
preparation example Construction
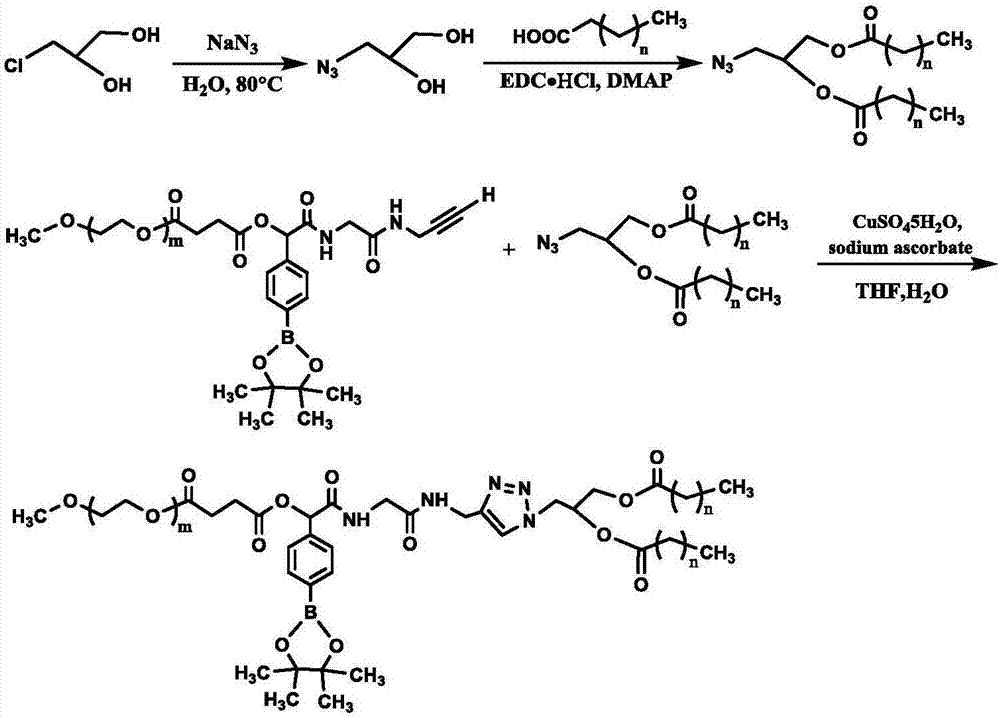
[0043] The present invention provides a method for preparing an oxidation-responsive PEGylated lipid material described in the above technical solution, comprising the following steps:
[0044] In the presence of a catalyst and a solvent, the polyethylene glycol monomethyl ether derivative having the structure of formula (II) and the azide diglyceride of fatty acid having the structure of formula (III) are click chemically reacted to obtain the structure of formula (I) Oxidation-responsive PEGylated lipid materials;
[0045]
[0046] In formula (II), 10≤m≤500;
[0047]
[0048] In formula (III), 0≤n≤20.
[0049] In the present invention, in order to distinguish it from the catalyst in the following technical scheme, the polyethylene glycol monomethyl ether derivative with the structure of formula (II) and the azidated fatty acid glyceride with the structure of formula (III) are clicked The catalyst used in the chemical reaction is named as the first catalyst. The firs...
Embodiment 1
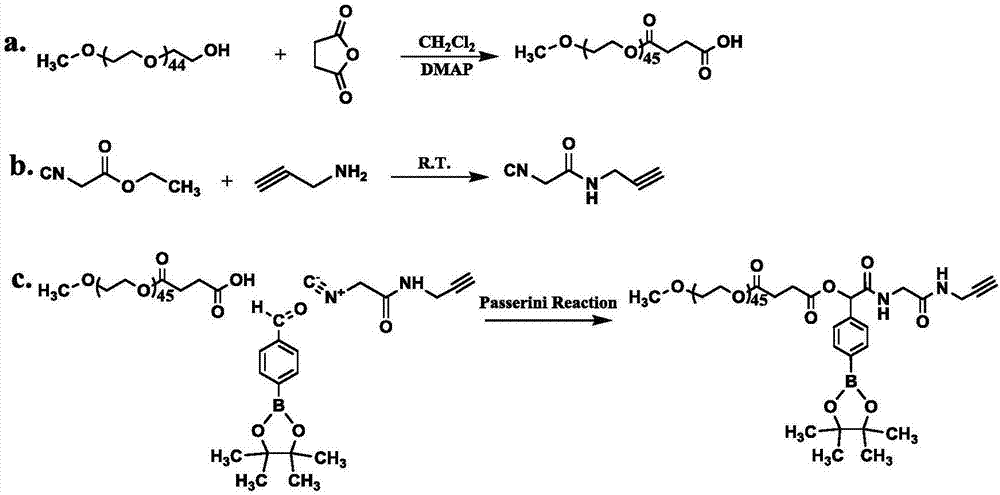
[0081] Synthesis of Carboxylated Polyethylene Glycol Monomethyl Ether
[0082] Weigh 7.5 g of polyethylene glycol monomethyl ether (molecular weight 750), 5 g of succinic anhydride, 244.3 mg of 4-dimethylaminopyridine, and 80 ml of dichloromethane, and add them into the reaction flask. Stir at room temperature for 48h. Part of the solvent was evaporated by rotary evaporation, and settled 3 times with anhydrous ether to obtain mPEG 0.75k -COOH.
Embodiment 2
[0083] Embodiment 2: the synthesis of the polyethylene glycol monomethyl ether of terminal carboxylation
[0084] Weigh 20 g of polyethylene glycol monomethyl ether (molecular weight: 2000), 5 g of succinic anhydride, 244.3 mg of 4-dimethylaminopyridine, and 120 ml of dichloromethane, and add them into the reaction flask. Stir at room temperature for 48h. Part of the solvent was evaporated by rotary evaporation, and settled 3 times with anhydrous ether to obtain mPEG 2k -COOH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com