Nano medicine delivery system for photo-thermal chemotherapy combined therapy and preparation method
A nano-drug delivery system and combined therapy technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of limiting ICG application and instability, and achieve simple synthesis methods Ease of application, wide application, and strong antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
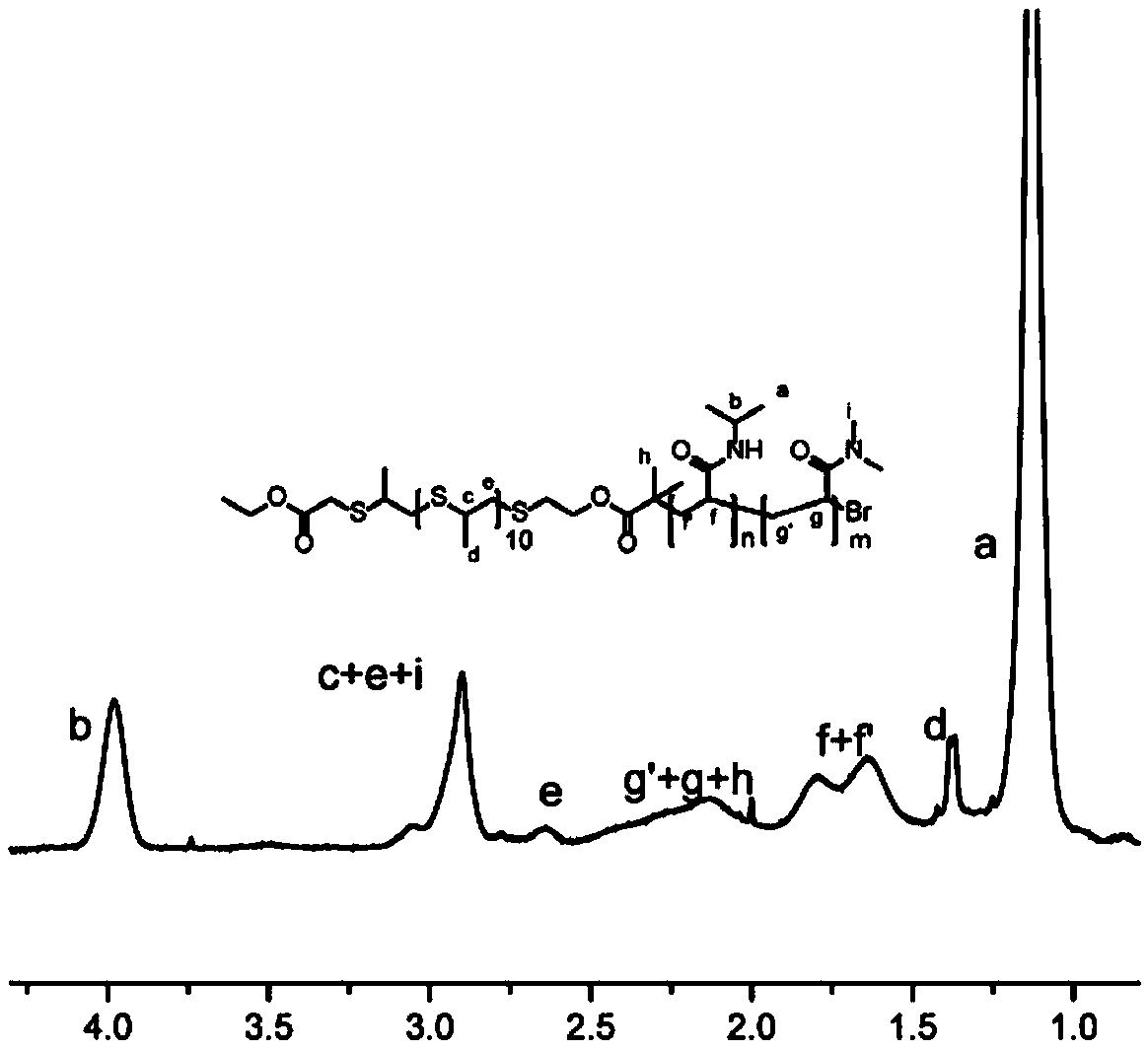
[0035] Copolymer preparation
[0036] Materials and Methods:
[0037] Propylene sulfide, purchased from TCI company, 2-bromo-2-methylpropionyl bromide, sodium methoxide methanol solution purchased from Bailingwei company, N-isopropylacrylamide, N,N-dimethylacrylamide purchased from Adamas company , Adriamycin was purchased from Anaiji Company, and indocyanine green was purchased from Macleans Company. The solvents used in the reaction were all re-evaporated to remove water and dried.
[0038] 1. Synthesis of polypropylene sulfide macroinitiator, its steps are:
[0039] Synthesized by anionic ring-opening polymerization method to prepare polypropylene sulfide. 20mL of dry tetrahydrofuran was introduced into nitrogen for 1h, then 2mL of TBP, 162mg of 2-hydroxyacetyl thioester, 2.8mL of sodium methoxide methanol solution were added to react for a few minutes, 1g of episulfide propene was added, and then reacted for a while, 0.6mL of bromoacetamide was added, React for 2 hours, spin off...
Embodiment 2
[0043] Preparation of drug-loaded micelles
[0044] Weigh 5mg DOX, 5mg ICG, and 10mg copolymer respectively, add appropriate amount of DMF to dissolve. Under constant stirring, slowly add to 10mL of water, continue to stir for 8h, and then dialyze to remove DMF solvent. After dialysis, the product is filtered through a 0.22μm microporous membrane for sterilization. The prepared micelles are named DIMs. In addition, micelles containing only DOX and only ICG were prepared according to the same method and named DMs and IMs, respectively.
Embodiment 3
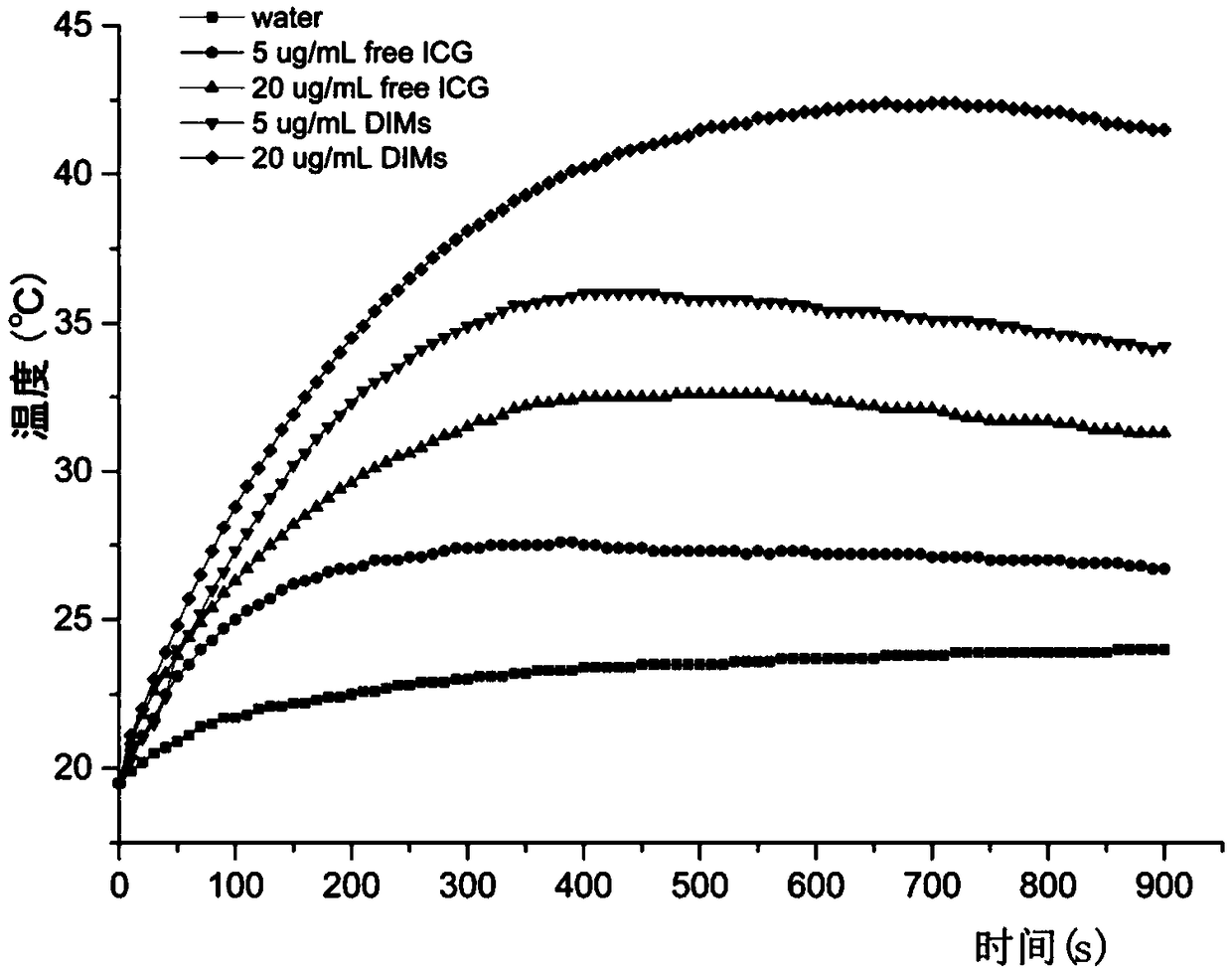
[0046] Photothermal experiment determination of drug-loaded micelles
[0047] Take 0.5mL of H 2 O, 5 and 20μg / mL ICG solution, 5 and 20μg / mL drug-loaded micelle solution, irradiate under 808nm near infrared light for 15min, and record the temperature change with a temperature recorder, such as image 3 As shown,
[0048] It can be seen that the maximum temperature of drug-loaded micelles is higher than that of LCST, which can cause the phase change of micelles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com