In-vitro expansion method, kit and application of umbilical cord blood NK (nature killer) cells
A technology for NK cells and in vitro expansion, applied in the field of cell engineering, can solve the problems of low yield of NK cells, high cost of separation and expansion, and poor safety, so as to simplify the cell preparation process, avoid safety risks, and reduce preparation costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1, in vitro expansion of umbilical cord blood NK cells and detection
[0080] 1. Expansion of umbilical cord blood NK cells in vitro (activation culture protocol 1)
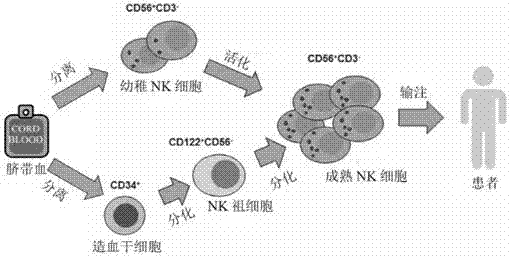
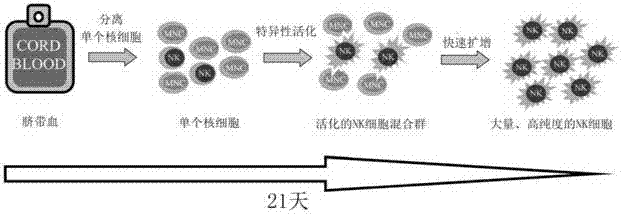
[0081] Such as figure 1 As shown, the in vitro expansion method of umbilical cord blood NK cells of the present invention comprises the following steps:
[0082] 1) Separation of umbilical cord blood mononuclear cells
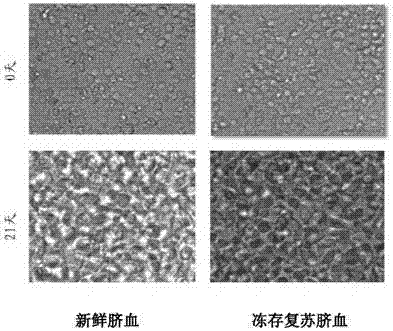
[0083] 1.1 Take 100mL of fresh anticoagulated cord blood (sample 1) and cryopreserved and resuscitated cord blood (sample 2, the cord blood is provided by the Obstetrics and Gynecology Department of the Armed Police General Hospital and approved by the Hospital Ethics Committee), and add 100mL of PBS (formula: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.24g, add distilled water to 1000mL, adjust pH 7.4) to dilute cord blood;
[0084] 1.2 Add 20mL of sample density separation solution (patent number: ZL201110456878.2, medical device record number: Jinghai Machine...
Embodiment 2
[0117] Embodiment 2, in vitro expansion of umbilical cord blood NK cells (activation culture scheme two)
[0118] Such as figure 1 As shown, the in vitro expansion of umbilical cord blood NK cells includes the following processes:
[0119] 1) Isolation of umbilical cord blood mononuclear cells from cryopreserved and resuscitated umbilical cord blood
[0120] Take 10ml of frozen and resuscitated umbilical cord blood (sample 3), the method is the same as in Example 1.
[0121] 2) activating and culturing umbilical cord blood NK cells (the control group is embodiment 1)
[0122] With GMP S&XFM TM - CD lymphocyte culture medium (patent application number: 201310082166.8; medical device record number: Jinghai Equipment No. 20150008) to adjust the mononuclear cell density to 2×10 6 A / mL (0.5~5×10 6 / mL is acceptable, preferably 1-3×10 6 cells / mL), the cell suspension was inoculated into cell culture flasks, and then injected into GMP S&XFM TM -Add 2μg / mL (1-10μg / mL, preferabl...
Embodiment 3
[0126] Example 3, in vitro expansion of umbilical cord blood NK cells and detection (activation culture scheme three)
[0127] Such as figure 1 As shown, the in vitro expansion of umbilical cord blood NK cells includes the following processes:
[0128] 1) Isolation of umbilical cord blood mononuclear cells from cryopreserved and resuscitated umbilical cord blood
[0129] Take 10ml of frozen and resuscitated umbilical cord blood (sample 3), the method is the same as in Example 1.
[0130] 2) Activation and culture of cord blood NK cells
[0131] With GMP S&XFM TM - CD lymphocyte culture medium (patent application number: 201310082166.8; medical device record number: Jinghai Equipment No. 20150008) to adjust the mononuclear cell density to 2×10 6 A / mL (0.5~5×10 6 / mL is acceptable, preferably 1-3×10 6 cells / mL), the cell suspension was inoculated into cell culture flasks, and then injected into GMP S&XFM TM - Add 2μg / mL (1-10μg / mL is acceptable, preferably 1-5μg / mL) zoled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com