Preparation of enzymatic chlorambucil-polypeptide hydrogel and application in resisting cancer
A technology for catalyzing chlorambucil and hydrogels, applied in the field of polypeptide hydrogels, can solve the problem that the influence of polypeptide folding, self-assembly and function is rarely studied, and achieve the effect of improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) According to Document 1, the phosphorylated polypeptide CRB-G was synthesized by FMOC-solid-phase synthesis method D f D f p D Y (ALP, 4°C), its nuclear magnetic spectrum and mass spectrometry results are as follows:
[0019] 1 H NMR (400MHz, DMSO) δ8.32(d, J=7.5Hz, 1H), 8.22(d, J=8.1Hz, 1H), 7.97(d, J=15.2Hz, 2H), 7.29-7.16(m , 9H), 7.04(dd, J=27.5, 8.1Hz, 3H), 6.65(d, J=8.3Hz, 2H), 4.55(d, J=8.9Hz, 1H), 4.50-4.36(m, 2H) , 3.67(d, J=12.4Hz, 5H), 3.53(dd, J=19.7, 12.5Hz, 2H), 3.47-3.38(m, 1H), 3.03(d, J=11.3Hz, 2H), 2.97- 2.88(m, 2H), 2.84-2.63(m, 3H), 2.54(s, 1H), 2.46-2.35(m, 2H), 2.08(t, J=7.3Hz, 2H), 1.77-1.64(m, 2H).M+=897.27, obsvd.(M+H) + =898.2741, (M+Na) + =920.2544.
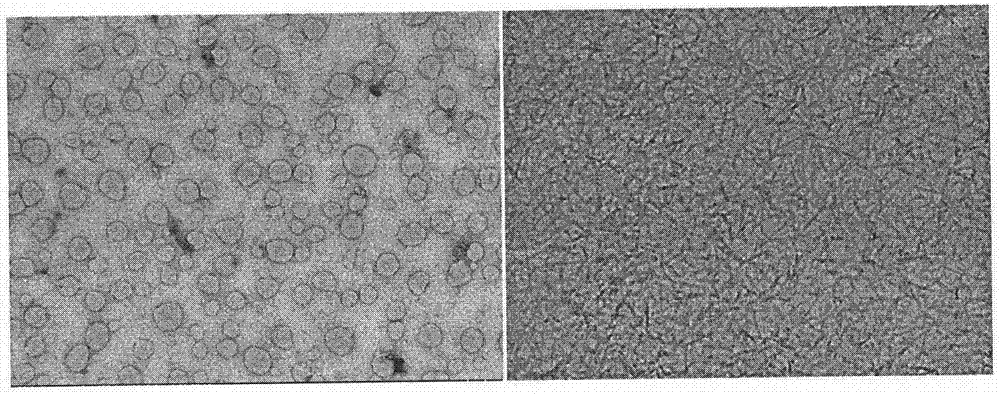
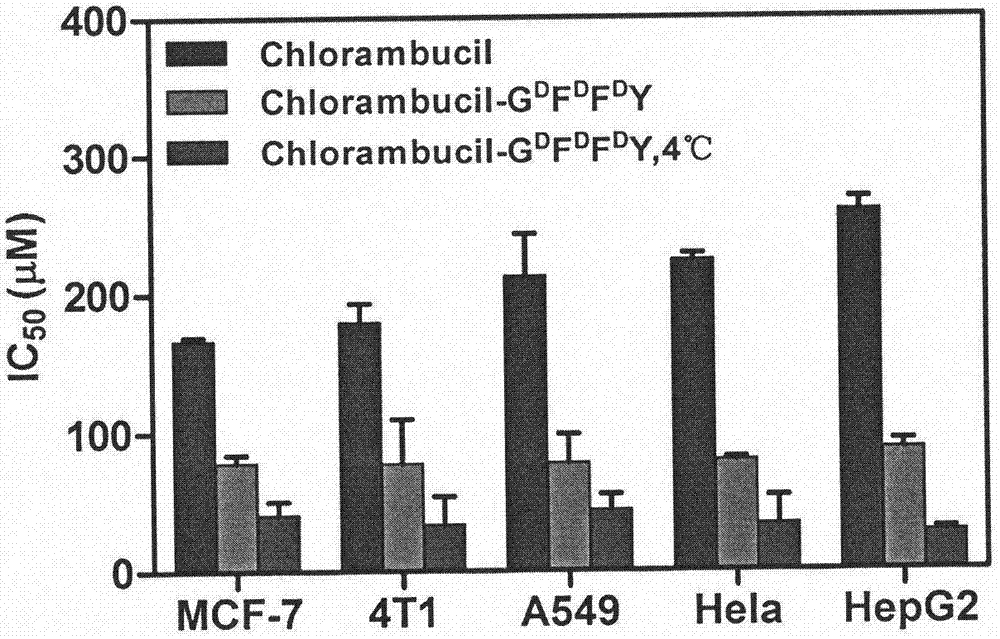
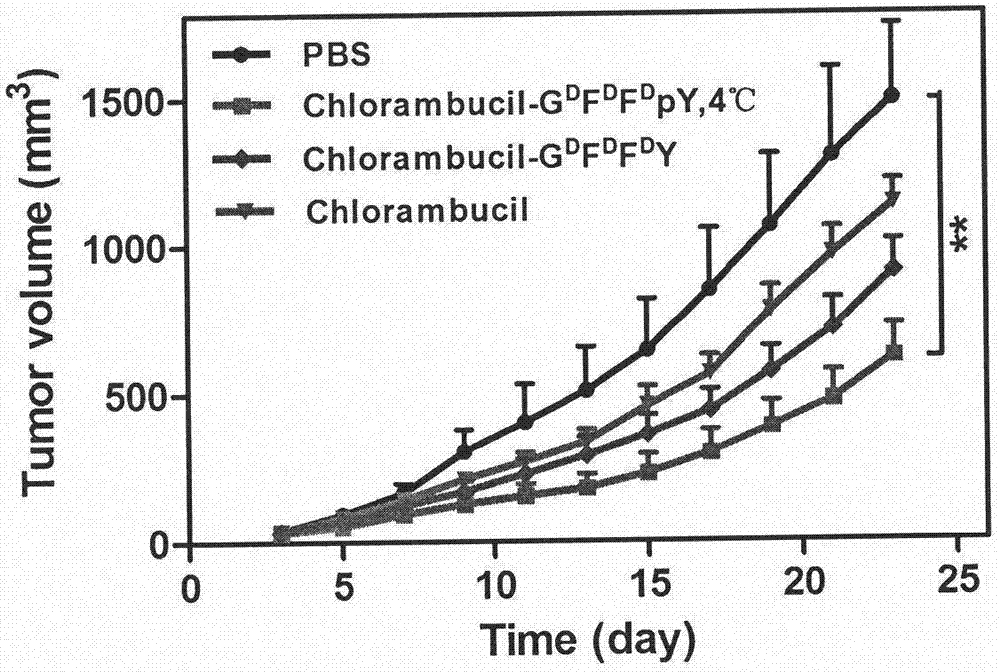
[0020] (2) Take 1 mg phosphorylated polypeptide CRB-G D f D f p D Y was placed in a 1.5 ml glass bottle, 990 microliters of PBS (pH=7.4) solution was added, the pH value was adjusted to 7.4 with sodium carbonate solution, 1 microliter of 1U / microliter alkaline phosphatase was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com