Synthesis of new 3,4,9,10-perylenetetracarboxylic diimide cyclometalated iridium complex and application to regulate fluorescence-phosphorescence dual emission through utilization of solution concentration of complex
A dual-emission, iridium complex technology, applied in indium organic compounds, platinum group organic compounds, compounds containing elements of Group 8/9/10/18 of the periodic table, etc., can solve problems such as unfavorable broad-spectrum emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] When R 1 When it is a methoxy group, taking the preparation of Ir-1, Ir-2 and Ir-4 as an example, the synthetic route is as follows:
[0039]
[0040] Synthesis of N,N'-di(dodecyl)-3,4,9,10-perylenetetracarboxyimide(1)
[0041] In a 100mL single-necked bottle, add 3,4,9,10-perylenetetracarboxylic dianhydride (1.0g, 2.55mmol), dodecylamine (2.8g, 15.31mmol), 5g imidazole and 5mL toluene in sequence, at 180°C Reaction 24h. Pour the reaction solution into 100mL 2M hydrochloric acid, filter with suction, and filter the cake with CH 2 Cl 2 As the eluent, it was separated by column chromatography to obtain 0.8 g of black-red solid with a yield of 43.2%. 1 H NMR (CDCl 3 ,400MHz,TMS),δ(ppm):8.67(d,J=7.6Hz,4H),8.60(d,J=8.0Hz,4H),4.21(t,J=6.8Hz,4H),1.76(m ,4H),1.46-1.26(m,36H),0.87(m,6H).
[0042] Synthesis of N,N'-bis(dodecyl)-1-nitro-3,4,9,10-perylenetetracarboxyimide (2)
[0043] In a 100mL three-necked flask, add N,N'-bis(dodecyl)-3,4,9,10-perylenetetracarboxylic d...
Embodiment 2
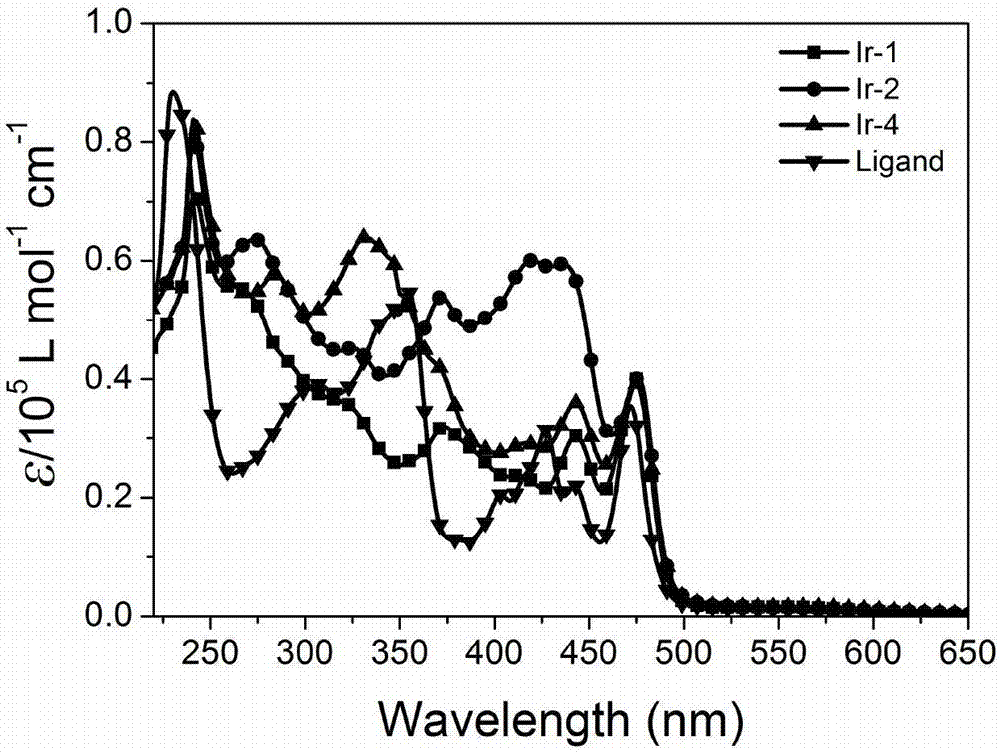
[0068] Perylene imide ligand and ring metal iridium complex ultraviolet performance test in embodiment 1:
[0069] The perylene imide auxiliary ligand and ionic cyclometal iridium complexes Ir-1, Ir-2 and Ir-4 were dissolved in chloroform solution (10 -5 mol / L), measure its ultraviolet absorption performance at room temperature, and its ultraviolet absorption spectrum is as figure 1 shown. Ligands and cyclometalated iridium complexes exhibit similar absorption spectra in the UV-vis region. According to similar literature reports, the absorption of strong absorption in the 240-580nm region is mainly attributed to the π-π allowed by the ligand spin * transitions, i.e. π-π including phenylpyridine * Transition, π-π of phenylisoquinoline * Transition, π-π of 4-[N,N-bis(4-methoxyphenyl)amino]phenyl-2-pyridine * Transition, π-π of 2-(4-dimethylborophenyl)pyridine * jump. The region from 530nm to 580nm shows a weaker absorption peak, which is mainly caused by the metal-to-liga...
Embodiment 3
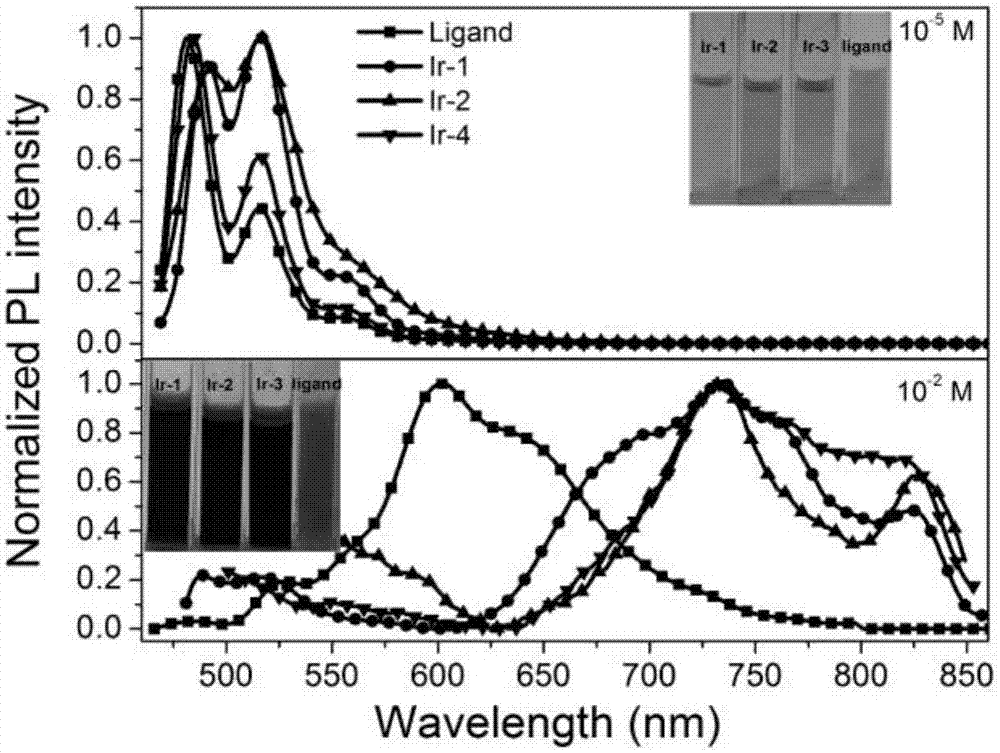
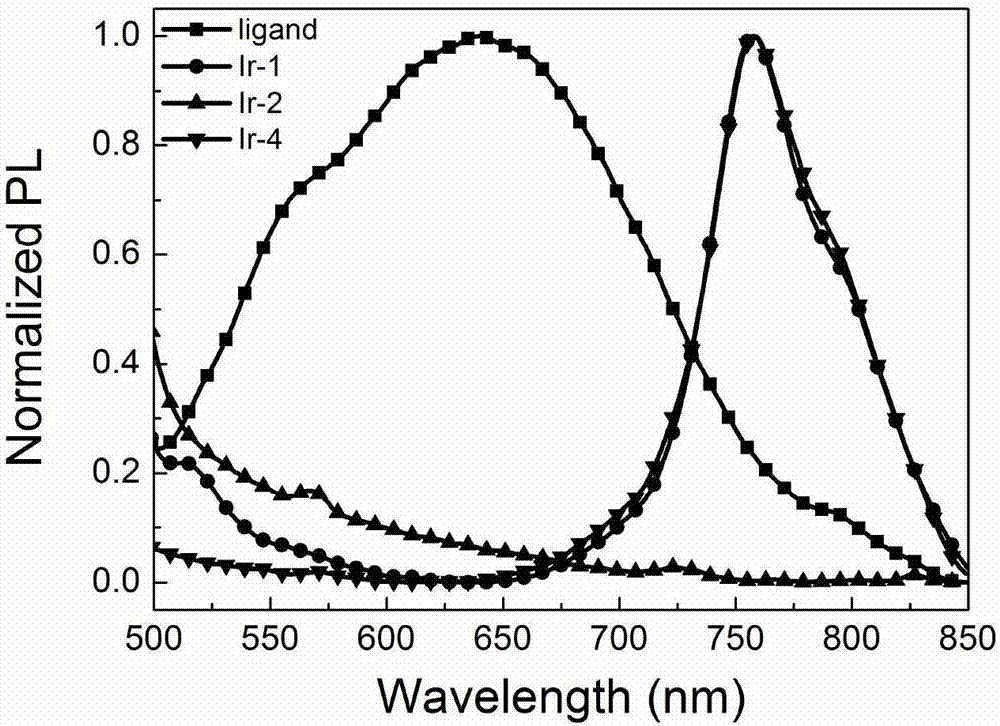
[0071] Photoluminescence performance test of perylene imide ligand and cyclometal iridium complex in Example 1:
[0072] The perylene imide ligand and the ring metal iridium complexes Ir-1, Ir-2 and Ir-4 were dissolved in chloroform, and the concentration was 1.0×10 -5 M and 1.0×10 -2 M solution, test its photoluminescence performance at room temperature, its photoluminescence spectrum is as follows figure 2 shown. When the excitation wavelength is 443nm, at low concentration (10 -5 M), the peryleneimide ligand has stronger emission at 481nm and 516nm ( figure 2 ). At the same time, the emission spectrum of the iridium complex is similar to that of the ligand, with a strong emission at 470-540nm, which is mainly attributed to the π-π transition of the peryleneimide ligand. At high concentrations (10 -2 M), the emission peak of the peryleneimide ligand is red-shifted by nearly 80nm compared with its low concentration, and there is a maximum emission at 600nm, which is m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence lifetime | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com