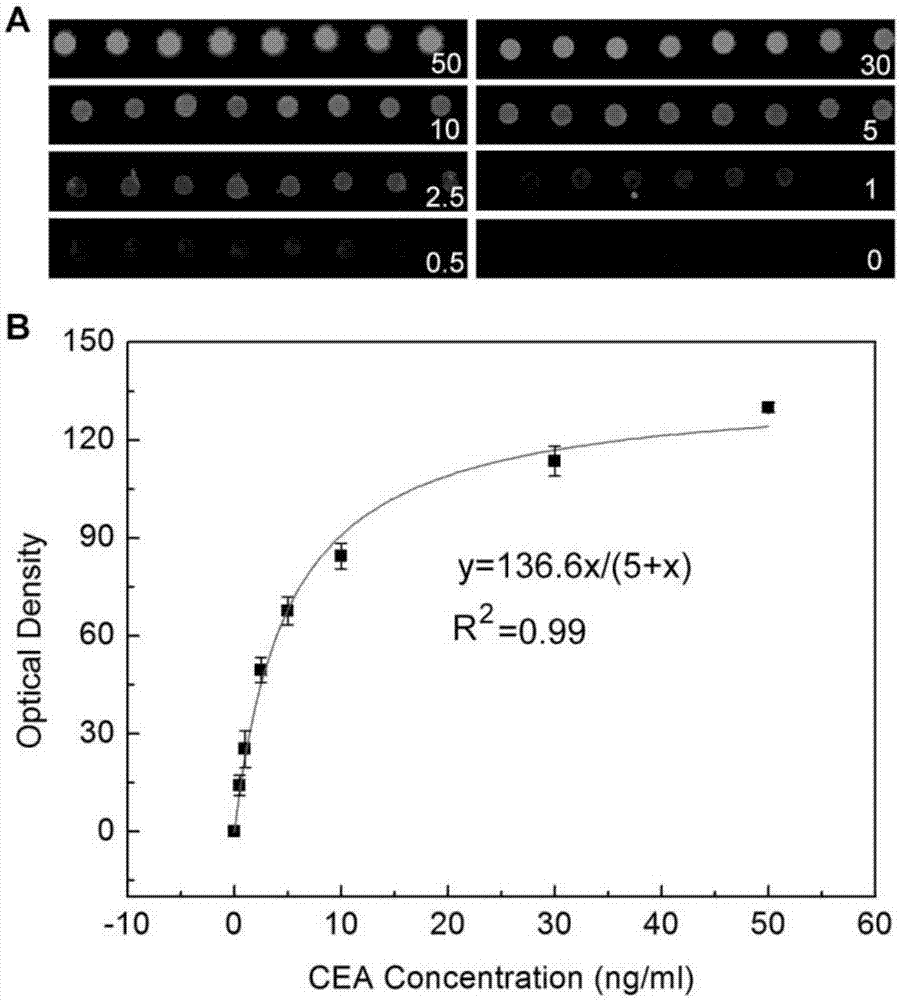
Microfluidic chip for detecting tumor markers based on uniformly distributed microspheres, and use method thereof
A microfluidic chip, tumor marker technology, applied in the field of biosensors, can solve problems such as obstruction of liquid flow, complicated experimental steps, unfavorable analysis, etc., to prevent clogging, simplify experimental operations, and avoid the effects of fluorescent signal interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
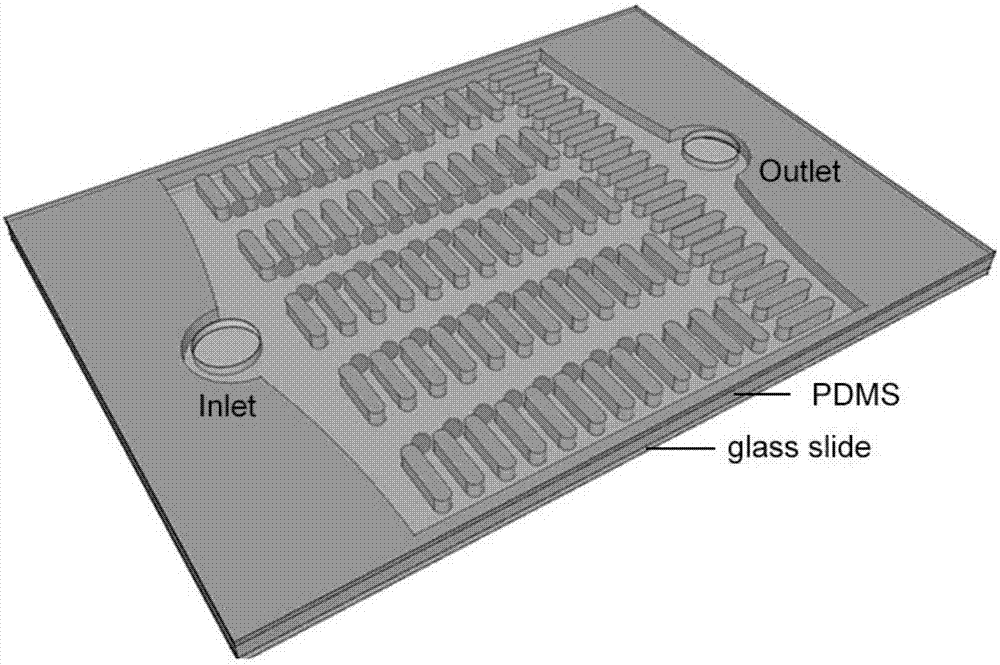
[0020] 1. Preparation of microfluidic chip
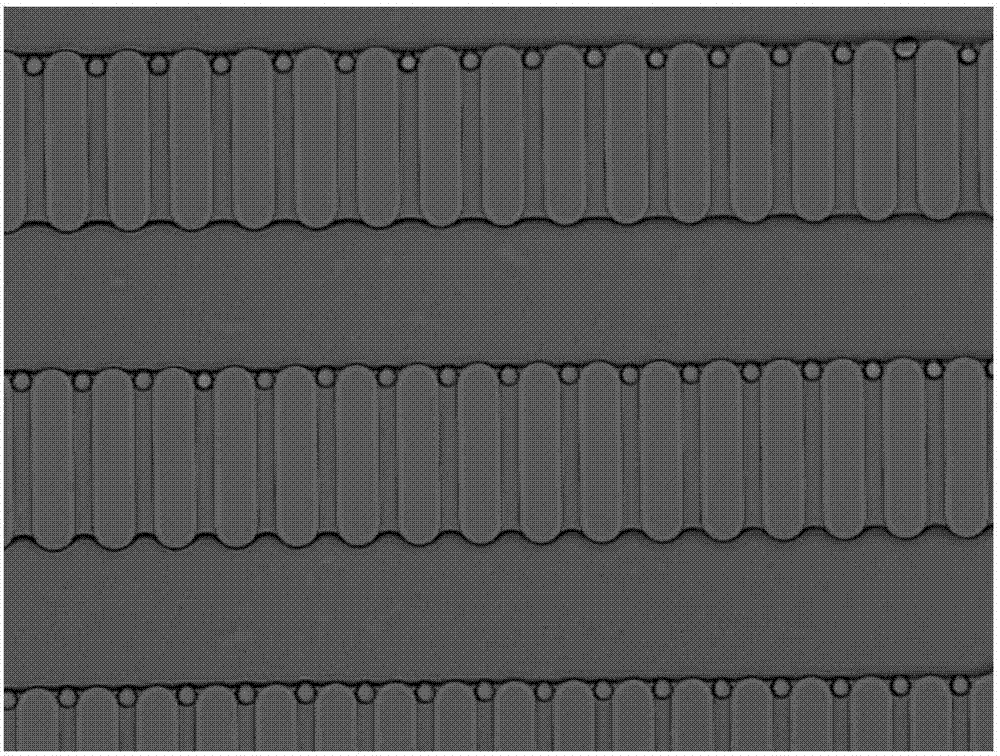
[0021] (1) Use CAD software to make the required mask. The length of the micro-pillars is 130 μm, the width is 30 μm, the narrowest spacing between the micro-pillars is 13 μm, and the distance between the micro-pillar arrays is 100 μm. The entire chip has about 25 μm. Each array has about 200 microcolumns, and the length of the microcolumns is perpendicular to the main flow direction.
[0022] (2) The photoresist is spin-coated on the cleaned silicon wafer with a thickness of 3 μm. After a series of photolithography processes such as exposure and development, the photoresist on the silicon wafer is patterned according to the pattern of the mask plate. Using the photoresist pattern after photolithography as a mask, carry out deep reactive ion etching on the silicon wafer, and the etching depth is 18 μm. This depth facilitates the microspheres to be arranged on a plane, which is conducive to focusing during observation. After the et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com