Time-resolved fluoroimmunoassay method for detecting avian influenza virus H7N9
A technology of time-resolved fluorescence and avian influenza virus, which is applied in the direction of antiviral immunoglobulin, analytical materials, immunoglobulin, etc., can solve the problem of lack of detection kits for H7N9, reduce the risk of H7N9 infection, be easy to promote, The effect of reducing economic loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of single-chain antibody phage library of highly pathogenic avian influenza virus H7N9, screening of single-chain antibody and induced expression
[0039] (1) Construction of anti-human avian influenza virus H7N9 single-chain antibody phage library
[0040] ① Isolation of total RNA: Human total mRNA was isolated from 10 mL of whole blood of a patient who had recovered from avian influenza H7N9 using a blood RNA extraction kit (Qiagen Corp).
[0041] ② Synthesize the first cDNA strands of IgG light and heavy chain genes of anti-human avian influenza virus H7N9 antibody: the RNA extracted in the above step is used as a template, and in order to increase the specificity of amplification, reverse transcription primers (HomoIgG1-4H1F, HomoCk-F and HomoCλ-F) to specifically amplify the first strand of cDNA of human IgG1 constant region and light chain k, lambda constant region.
[0042] The above reverse transcription primer sequence is:
[0043] Homo...
Embodiment 2
[0075] Example 2 Time-resolved fluorescent immunoassay kit for highly pathogenic avian influenza virus H7N9
[0076]The time-resolved fluorescent immunoassay kit for H7N9 contains the following components:
[0077] (1) Anti-H7N9 single-chain antibody
[0078] The anti-H7N9 single-chain antibody was selected from one of the single-chain antibodies A1, B7, and F10 described in Experimental Example 1 above.
[0079] (2) Enzyme-linked plates coated with antibodies
[0080] Use 50mmol / L, pH 9.6 carbonate buffer to dilute the coating antibody B7 (A1 or F10 can also be used) to 1-5μg / ml, then add 100μl / well to each well of the Elisa plate, 4 overnight at ℃, discard the coating solution, add blocking solution, 200 μl / well, block overnight, discard the coating solution, wash and pat dry, and store in vacuum at -20℃.
[0081] (3) Eu 3+ labeled antibody
[0082] Add 0.5 mg of the labeled antibody into a centrifuge tube with a filter membrane from Millipore, and centrifuge at 8000 r / ...
Embodiment 3
[0091] Example 3 Time-resolved fluorescent immunoassay method for highly pathogenic avian influenza virus H7N9
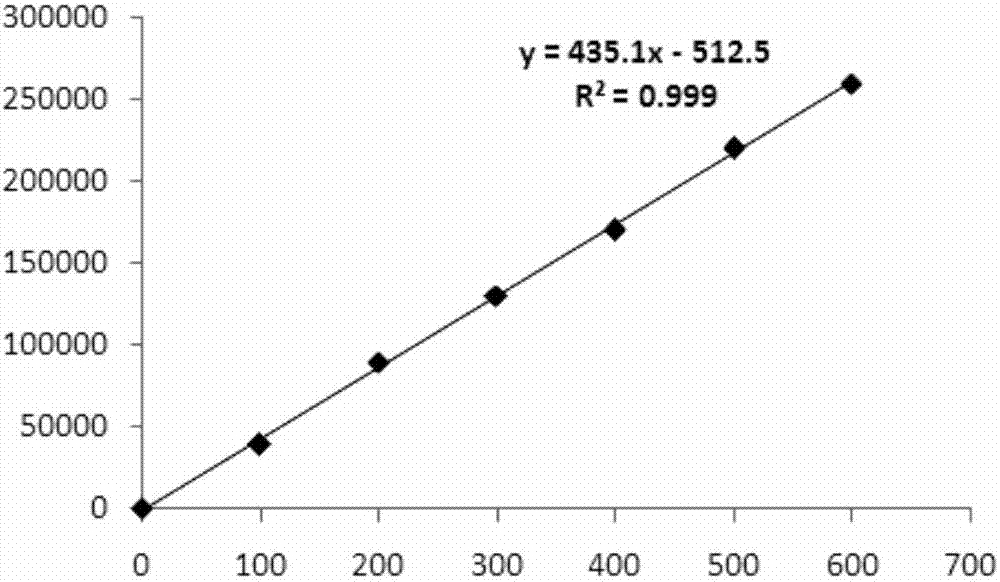
[0092] The operation steps of the time-resolved fluorescent immunoassay kit for quantitative detection of H7N9 virus of the present invention are as follows: add 25 μl of reference standard or sample to be tested to the coated enzyme-linked plate, then add 200 μl of analysis buffer, incubate with shaking for 1 hour, wash with Wash 4 times, use the analysis buffer to dilute the Eu 3+ Label the antibody to 1-5 μg / ml, add 200 μl / well, incubate with shaking for 1 h, wash with washing solution 6 times, and finally add 200 μl / well of enhancement solution (Perkin Elmer Company) and shake for 5 minutes before detecting on a time-resolved fluorescence detector.
[0093] The effect detection of the kits prepared in the above examples is performed below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com