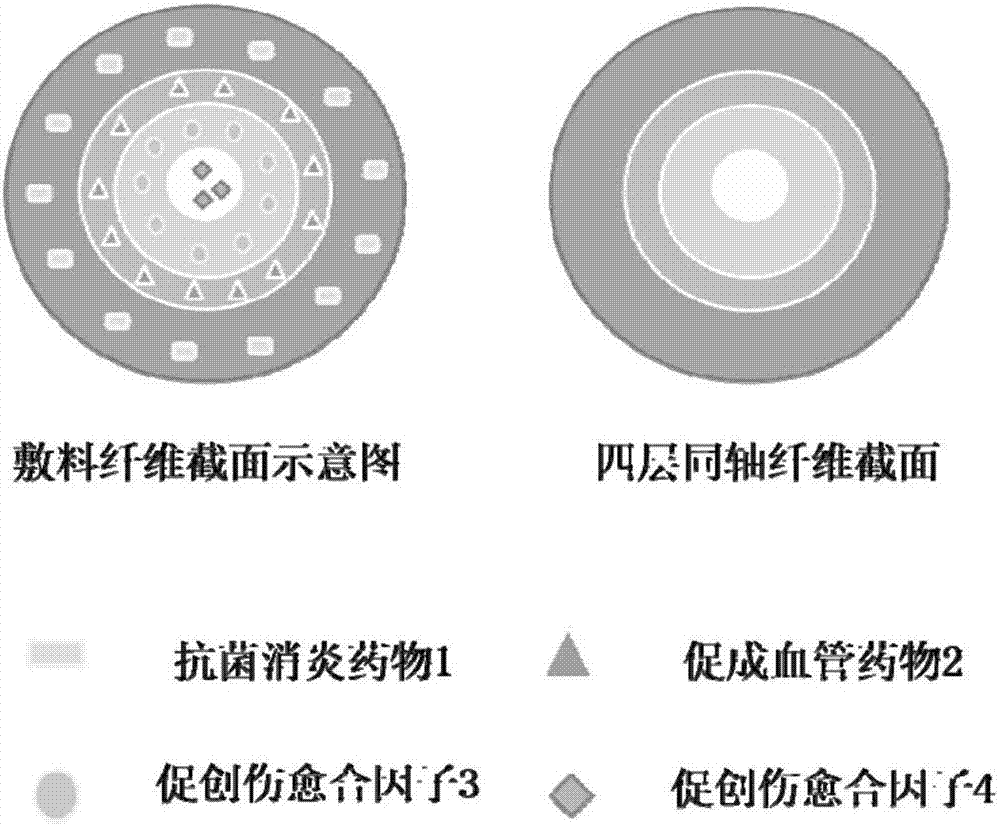
Four-layer coaxial fiber wound dressing and preparation method thereof
A wound dressing and fiber technology, which can be applied in fiber processing, fiber chemical characteristics, cellulose/protein conjugated artificial filaments, etc., can solve the problems of drug burst release phenomenon and short drug release time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) Take 2 g of gelatin, add it to 10 ml of trifluoroethanol solvent, and stir magnetically at room temperature for 9 hours to obtain a solution A with a gelatin mass concentration of 0.2 g / ml;
[0044] (2) Add 2×10 -2 g penicillin, magnetically stirred at room temperature for 3h to obtain solution B, the mass ratio of penicillin and gelatin in solution B is 1 / 100;
[0045] (3) Take 0.5 g of polycaprolactone, add it to 10 ml of trifluoroethanol solvent, and stir magnetically at room temperature for 10 hours to obtain a solution C with a mass concentration of polycaprolactone of 0.05 g / ml;
[0046] (4) Add the gelatin of 1.5g in solution C, magnetically stir at room temperature 9h, the solution D that the total mass concentration that obtains polycaprolactone and gelatin is 0.2g / ml, the mass ratio of polycaprolactone and gelatin in solution D for 25 / 75;
[0047] (5) Add 2×10 to solution D -3 g desferroxamine (DFO), magnetically stirred at room temperature for 6h, to o...
Embodiment 2
[0056] (1) Take 0.5 g of polycaprolactone, add it to 10 ml of trifluoroethanol solvent, and stir magnetically at room temperature for 10 hours to obtain a solution A with a mass concentration of polycaprolactone of 0.05 g / ml;
[0057] (2) Add 1.5g gelatin in solution A, magnetically stir at room temperature 9h, obtain the solution B that the total mass concentration of polycaprolactone and gelatin is 0.20g / ml, the mass ratio of polycaprolactone and gelatin in solution B is 25 / 75;
[0058] (3) Add 0.4g cephalosporin to solution B, stir magnetically at room temperature for 1h to obtain solution C, the ratio of the total mass of cephalosporin to polycaprolactone and gelatin in solution C is 20 / 100;
[0059] (4) Take 1.0 g of polycaprolactone, add it to 9.5 ml of trifluoroethanol solvent, and stir magnetically at room temperature for 10 h to obtain solution D;
[0060] (5) Add 1.0 g of gelatin to solution D, and magnetically stir at room temperature for 9 hours to obtain solution...
Embodiment 3
[0070] (1) Take 0.5 g of polylactic acid, add 10 ml of N,N-dimethylformamide solvent, and magnetically stir at room temperature for 9 hours to obtain solution A with a mass concentration of polylactic acid of 0.02 g / ml;
[0071](2) Add the chitosan of 1.5g in solution C, magnetically stir 10h at room temperature, obtain solution B, the mass ratio of polylactic acid and chitosan in solution B is 25 / 75;
[0072] (3) Add 0.04g tetracycline to solution B, magnetically stir at room temperature for 6h, obtain solution C, the ratio of the quality of tetracycline in solution C to the total mass of polylactic acid and chitosan is 2 / 100;
[0073] (4) Take 1 g of polylactic acid, add 10 ml of N,N-dimethylformamide solvent, and magnetically stir at room temperature for 9 hours to obtain a solution D with a mass concentration of polylactic acid of 0.10 g / ml;
[0074] (5) Add the chitosan of 1g in D solution, magnetically stir 10h at room temperature, obtain solution E, the mass ratio of po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com