Method for preparing hydrogen by reducing carbon dioxide by using alkali metal hydride
A technology of alkali metal hydride and carbon dioxide, applied in the field of clean energy, can solve problems such as unfavorable M-N-H system applications, and achieve the effect of simple reaction preparation equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
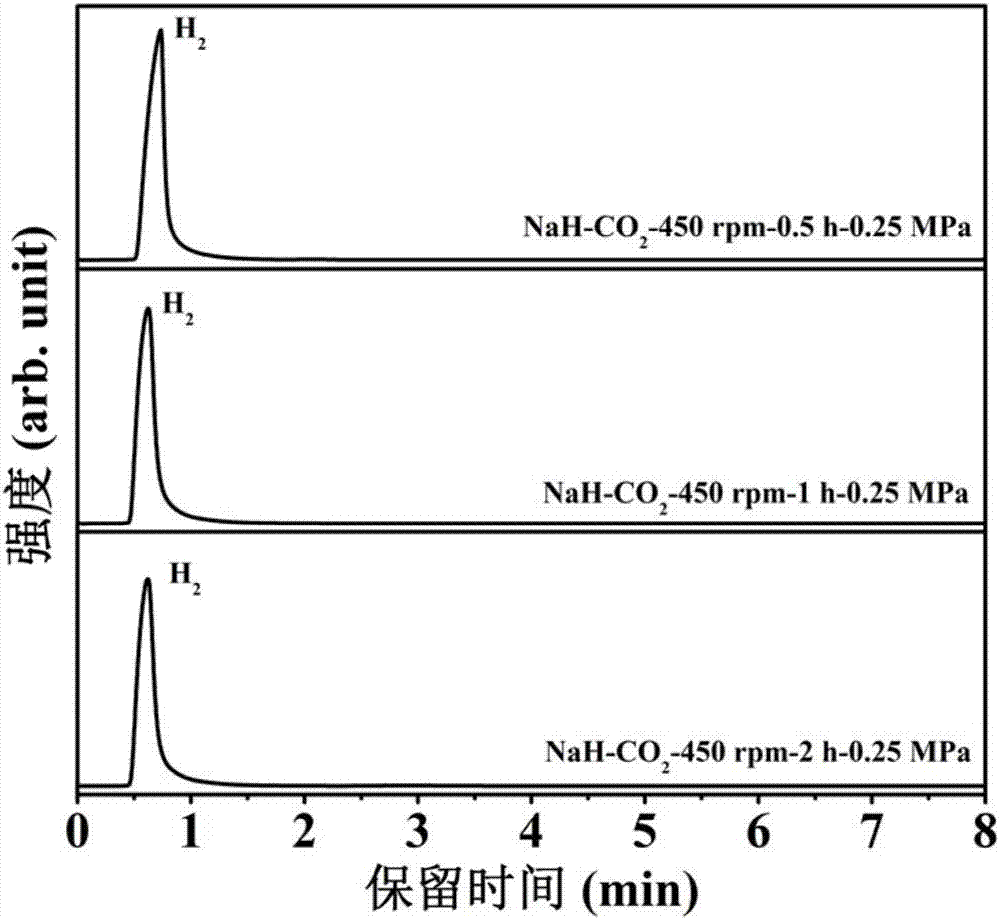
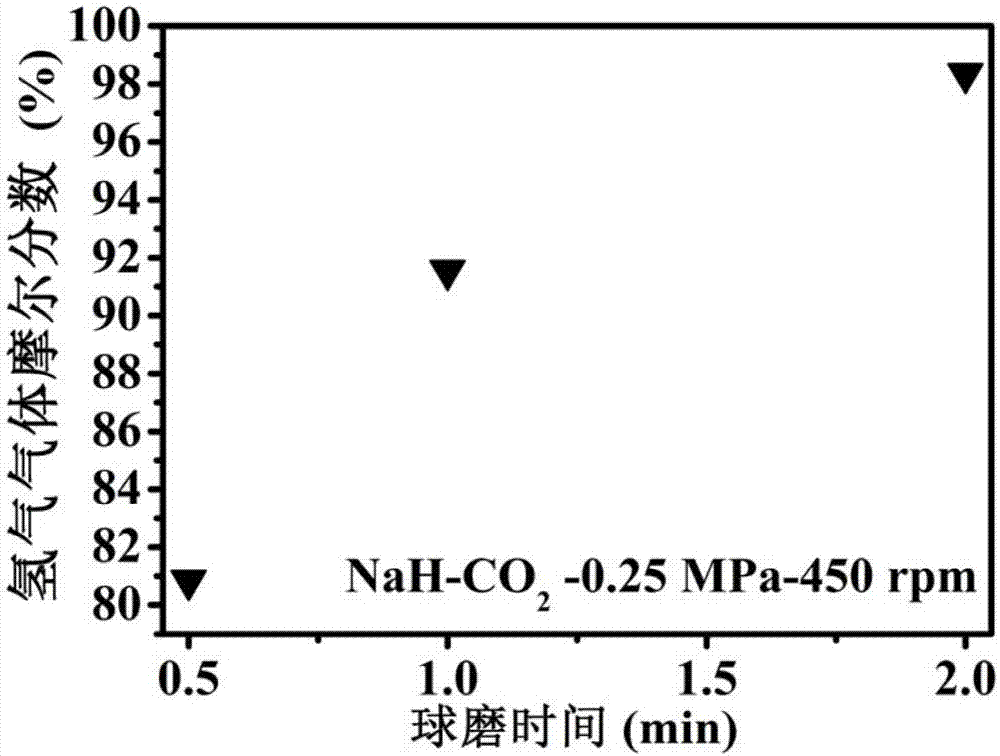
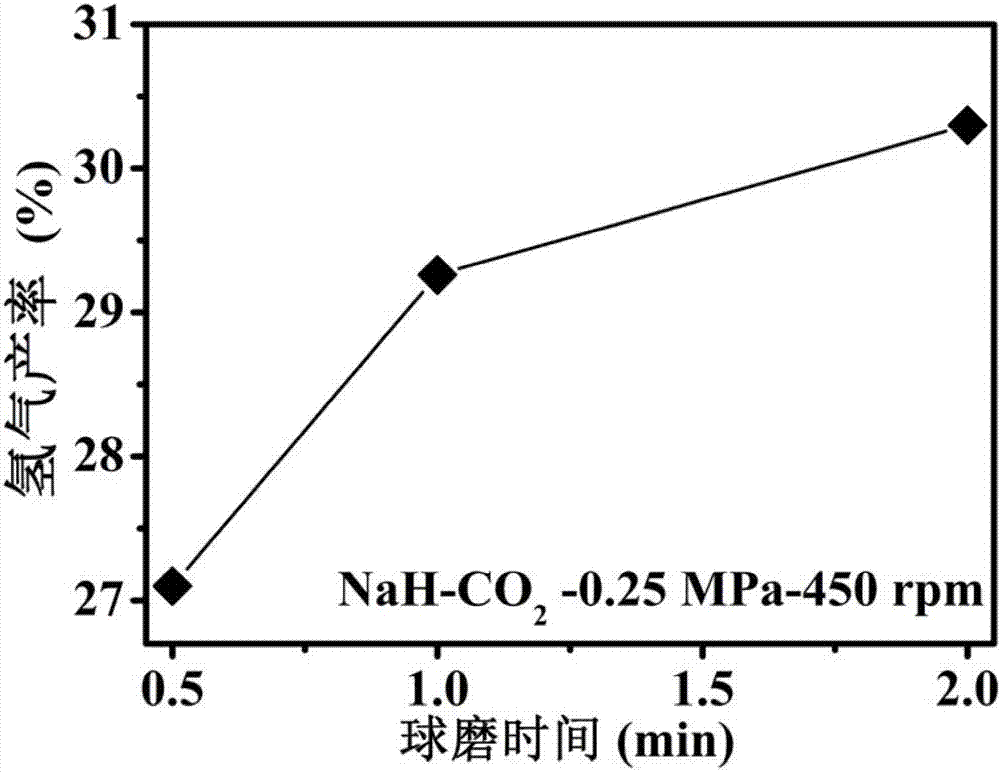
[0031] 1. In an argon glove box, place the NaH sample in a ball mill jar (with an internal volume of about 70 cm 3 ), put 30 small steel balls (diameter 6mm), take out the ball mill tank containing the NaH sample, pump out the argon gas and fill it with 0.25MPa high-purity CO 2 gas such that NaH / CO 2 The molar ratio is 4:1, using a planetary ball mill (QM-3SP4) at a speed of 450 rpm to perform ball milling reactions for 0.5h, 1h, and 2h respectively to obtain a mixed gas.
[0032] 2. After the reaction is over, the generated gas is passed into a vacuum pipeline system with a pressure sensor and connected to the chromatogram for GC detection. The product is characterized by the peak position, the peak area, and the pressure of the mixed gas after the reaction to calculate the hydrogen in the mixture. The mole fraction in the gas and the yield of hydrogen after the reaction.
Embodiment 2
[0034] 1. In an argon glove box, place the KH sample in a ball mill jar (with an internal volume of about 70 cm 3 ), put 30 small steel balls (diameter 6mm), take out the ball mill tank containing the KH sample, pump out the argon and fill it with 0.25MPa high-purity CO 2 gas such that KH / CO 2 The molar ratio is 4:1, using a planetary ball mill (QM-3SP4) at a speed of 450 rpm to perform ball milling reactions for 0.5h, 1h, and 2h respectively to obtain a mixed gas.
[0035] 2. After the reaction is over, the generated gas is passed into a vacuum pipeline system with a pressure sensor and connected to the chromatogram for GC detection. The product is characterized by the peak position, the peak area, and the pressure of the mixed gas after the reaction to calculate the hydrogen in the mixture. The mole fraction in the gas and the yield of hydrogen after the reaction.
[0036] Three, the yield calculation method of hydrogen in the mixed gas that each example obtains:
[0037]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com