Method of using alkaline earth metal hydrides to prepare hydrogen-methane mixed fuel
An alkaline earth metal, mixed fuel technology, applied in the production of hydrocarbons from carbon oxides, hydrogen production, organic chemistry, etc., can solve the problems of incomprehensible reaction mechanism, expensive equipment, complicated operation, etc., to reduce combustion duration, reduce The effect of quenching interval and simple preparation device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
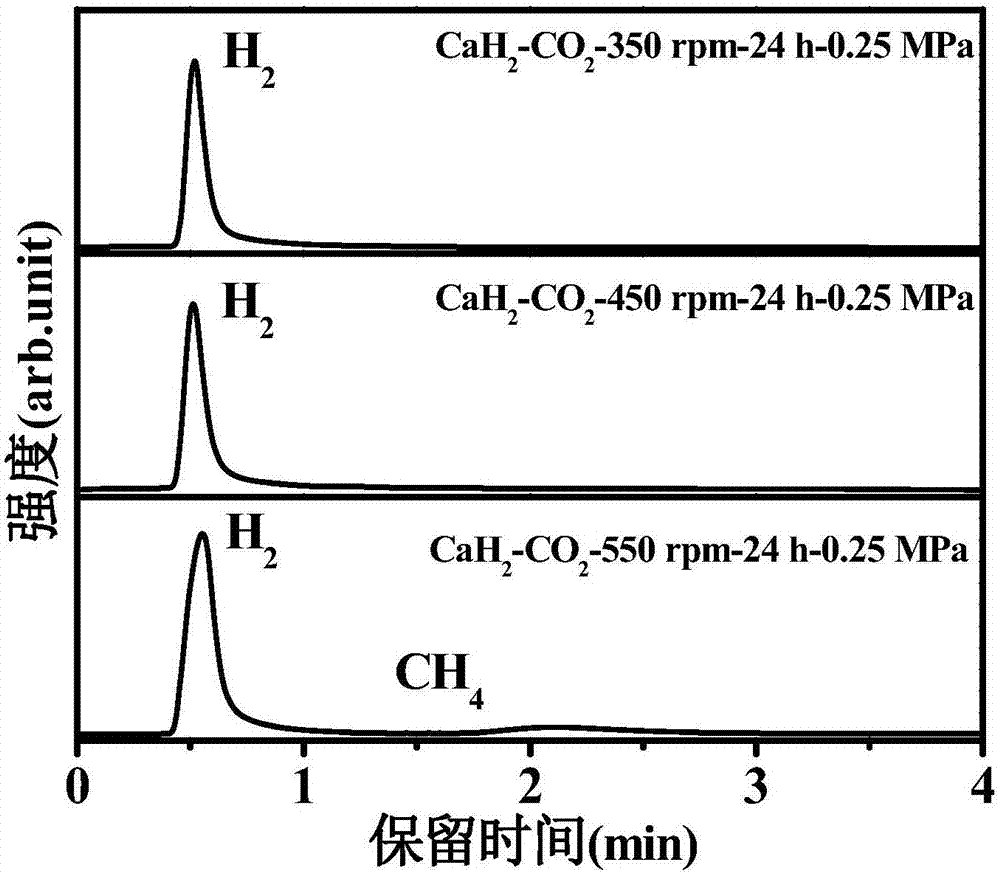
[0026] 1. In an argon glove box, the CaH 2 The sample is placed in a ball mill jar (with an internal volume of about 70 cm 3 ), put 30 small steel balls (diameter 6mm), take out the CaH 2 The ball mill tank of the sample is filled with 0.25MPa high-purity CO after the argon gas is pumped out. 2 gas, such that CaH 2 / CO 2 The molar ratio is 2:1, and the planetary ball mill (QM-3SP4) is used to carry out the ball milling reaction at 350 rpm, 450 rpm, and 550 rpm for 24 hours to obtain a mixed gas of methane and hydrogen.
[0027] 2. After the reaction is over, the generated gas is passed into a vacuum pipeline system with a pressure sensor and connected to the chromatogram for GC detection. The product is characterized by the peak position, the peak area, and the pressure of the mixed gas after the reaction to calculate the methane in the mixture. The mole fraction in the gas and the yield of methane after the reaction.
Embodiment 2
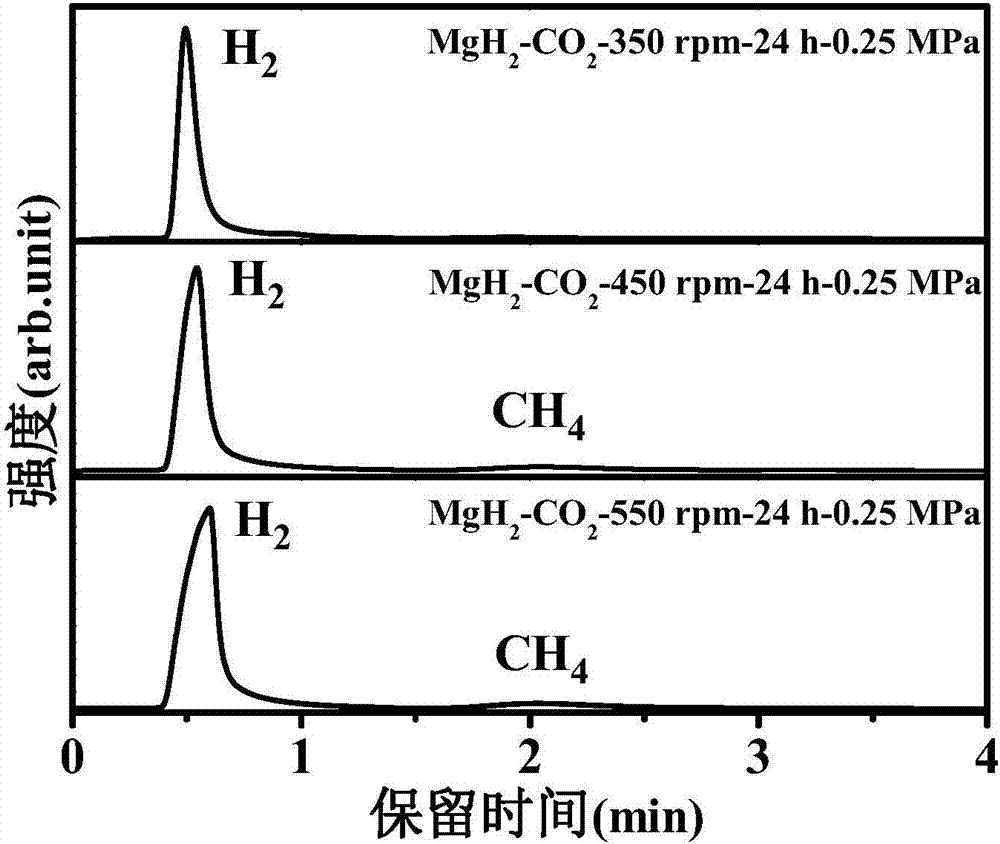
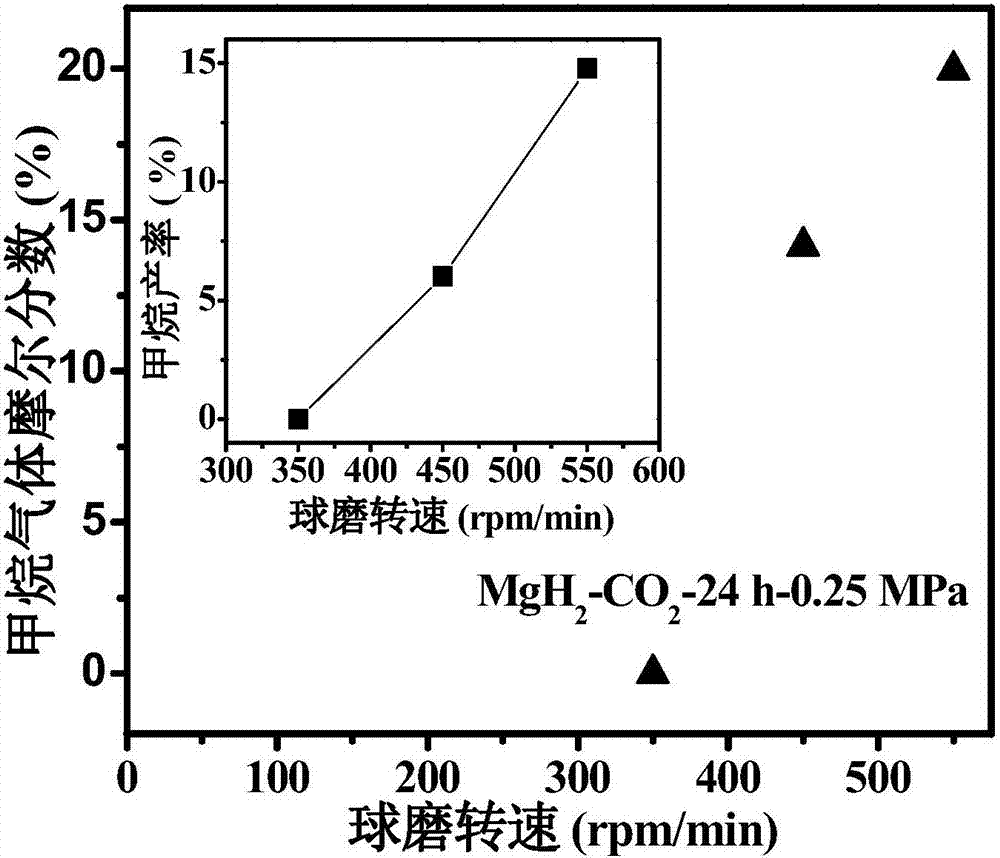
[0029] 1. Dissolve MgH in an argon glove box 2 The sample is placed in the ball milling jar, the ball milling jar is taken out, the argon in it is pumped out and filled with 0.25MPa high-purity CO 2 gas, such that MgH 2 / CO 2 The molar ratio is 2:1, and the planetary ball mill (QM-3SP4) is used to carry out the ball milling reaction at 350 rpm, 450 rpm, and 550 rpm for 24 hours to obtain a mixed gas of methane and hydrogen.
[0030] 2. After the reaction is over, the generated gas is passed into a vacuum pipeline system with a pressure sensor and connected to the chromatogram for GC detection. The product is characterized by the peak position, the peak area, and the pressure of the mixed gas after the reaction to calculate the methane in the mixture. Graph of the mole fraction in the gas and the yield of methane after the reaction.
Embodiment 3
[0032] 1. Dissolve MgH in an argon glove box 2 The sample is placed in the ball mill jar, and the ball mill jar is taken out, and the argon gas in it is pumped out and then filled with 0.1MPa, 0.25MPa, and 0.5MPa high-purity CO 2 gas, and makes MgH 2 / CO 2The molar ratio is 2:1, and the planetary ball mill (QM-3SP4) is used to carry out the ball milling reaction at a speed of 450 rpm for 24 hours to obtain a mixed gas of methane and hydrogen.
[0033] 2. After the reaction is over, the generated gas is passed into a vacuum pipeline system with a pressure sensor and connected to the chromatogram for GC detection. The product is characterized by the peak position, the peak area, and the pressure of the mixed gas after the reaction to calculate the methane in the mixture. Graph of the mole fraction in the gas and the yield of methane after the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com