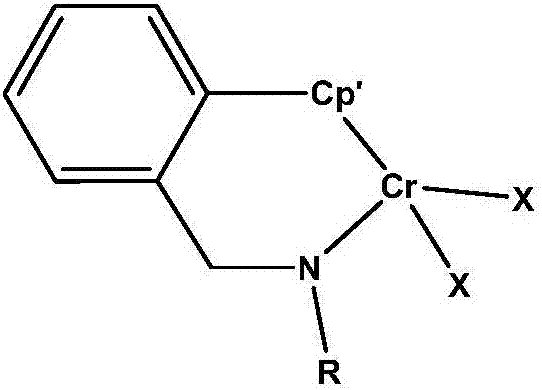
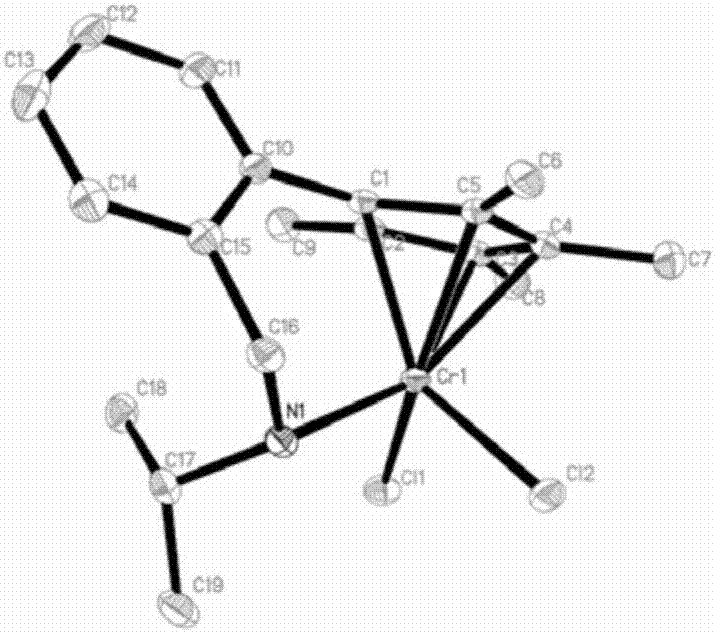
Benzylamine side arm-containing half-metallocene chromium (IV) complex and application thereof
A single metallocene, benzylamine group-containing technology, applied in the application of catalyzing ethylene homopolymerization and ethylene/α-olefin copolymerization, in the field of benzylamine sidearm single metallocene chromium complexes, which can solve problems such as synthesis difficulties , to achieve the effect of high catalytic activity, moderate copolymerization performance and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
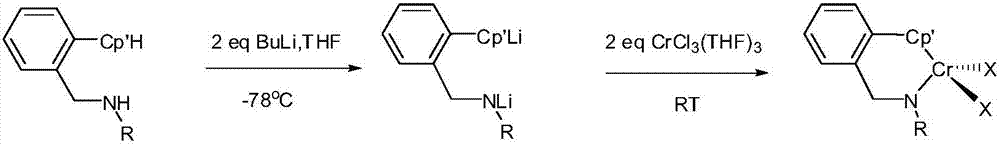
[0022] The preparation of embodiment 1 complex 1
[0023]Under nitrogen protection, 2-(isopropyl-nitrogen-methyl)phenyl-tetramethylcyclopentadiene (ligand 1) (0.700g, 2.60mmol) in tetrahydrofuran (20mL ) solution was added dropwise a n-hexane solution of n-butyllithium (2.16mL, 5.20mmol). After the addition was complete, the resulting reaction mixture was stirred at this temperature for an additional hour, then the mixture was allowed to rise to room temperature and continued stirring at this temperature for three hours, which was slowly added to 2 equivalents of CrCl 3 (THF) 3 in tetrahydrofuran solution, stirred overnight, removed the solvent, added 20 mL of toluene to the ampoule, filtered to remove insoluble matter, and removed the toluene in the filtrate in vacuo. Then 20 mL of hexane was added to the ampoule for ultrasonic solidification and filtered. The obtained solid was washed with cold hexane (5 mL×2), and then recrystallized with THF / hexane. 0.541 g of the pure ...
Embodiment 2
[0025] The preparation of embodiment 2 complex 2
[0026] Under nitrogen protection, at -78°C, 2-(cyclohexyl-nitrogen-methyl)phenyl-tetramethylcyclopentadiene (ligand 2) (0.805g, 2.60mmol) in tetrahydrofuran (20mL) A solution of n-butyllithium (2.16 mL, 5.20 mmol) in n-hexane was added dropwise to the solution. After the addition was complete, the resulting reaction mixture was stirred at this temperature for an additional hour, after which the mixture was allowed to warm to room temperature and stirred at this temperature for an additional three hours. Add it to 2 equivalents of CrCl 3 (THF) 3 The tetrahydrofuran solution was stirred overnight, the solvent was removed, 20 mL of toluene was added to the ampoule, the insoluble matter was removed by filtration, and the toluene in the filtrate was removed in vacuo. Then 20 mL of hexane was added to the ampoule for ultrasonic solidification and filtered. The obtained solid was washed with cold hexane (5 mL×2), and then recrysta...
Embodiment 3
[0027] The preparation of embodiment 3 complex 3
[0028] Under nitrogen protection, at -78°C, 2-(phenyl-nitrogen-methyl)phenyl-tetramethylcyclopentadiene (ligand 3) (0.789g, 2.60mmol) in tetrahydrofuran (20mL) A solution of n-butyllithium (2.16 mL, 5.20 mmol) in n-hexane was added dropwise to the solution. After the addition was complete, the resulting reaction mixture was stirred at this temperature for an additional hour, after which the mixture was allowed to warm to room temperature and stirred at this temperature for an additional three hours. Add it to 2 equivalents of CrCl 3 (THF) 3 The tetrahydrofuran solution was stirred overnight, the solvent was removed, 20 mL of toluene was added to the ampoule, the insoluble matter was removed by filtration, and the toluene in the filtrate was removed in vacuo. Then 20 mL of hexane was added to the ampoule for ultrasonic solidification and filtered. The obtained solid was washed with cold hexane (5 mL×2), and then recrystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com