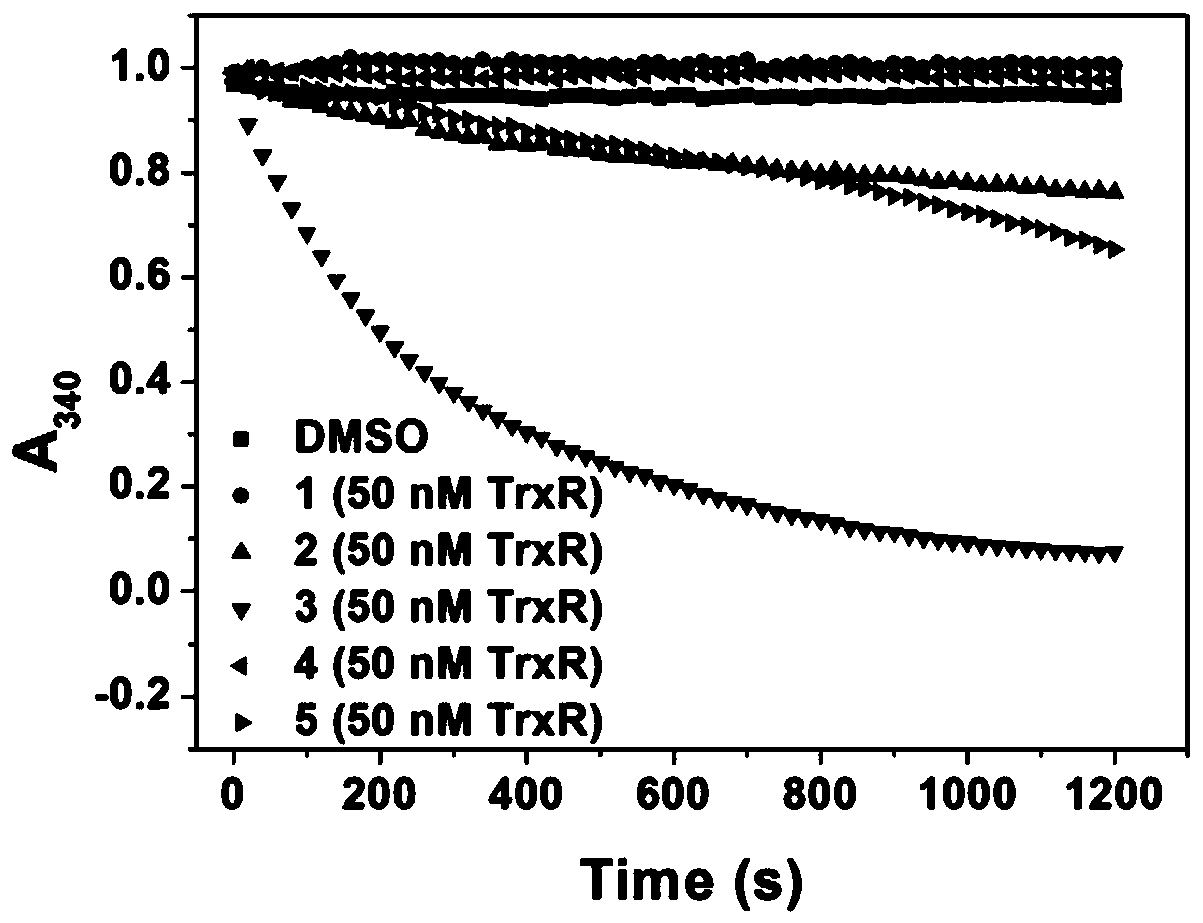
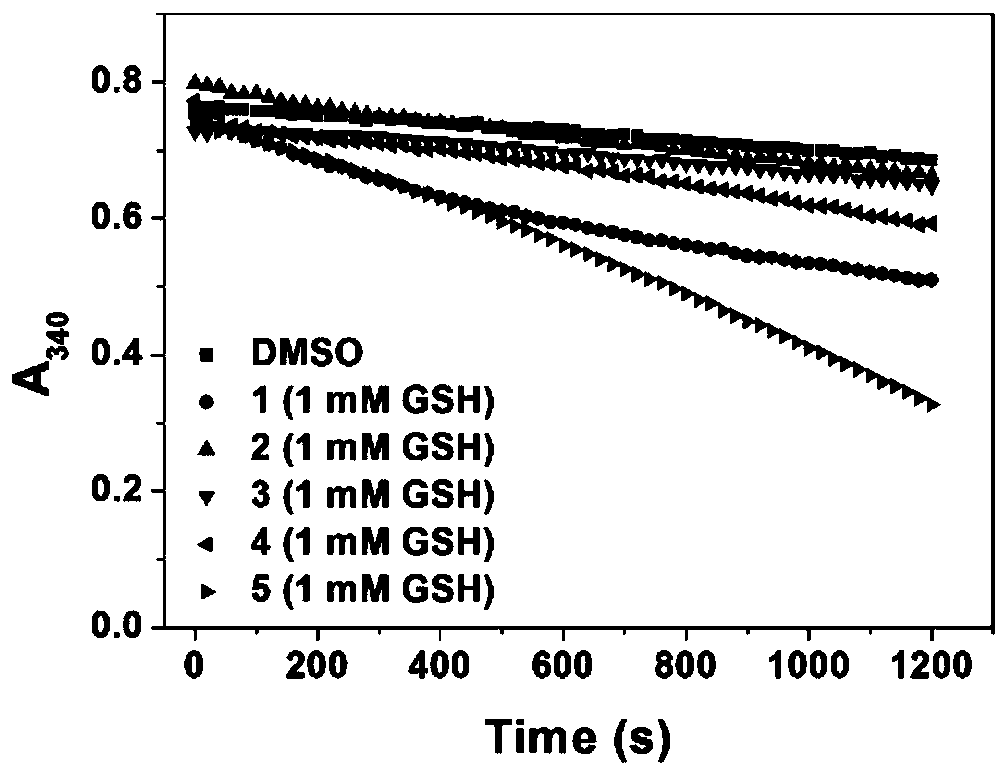
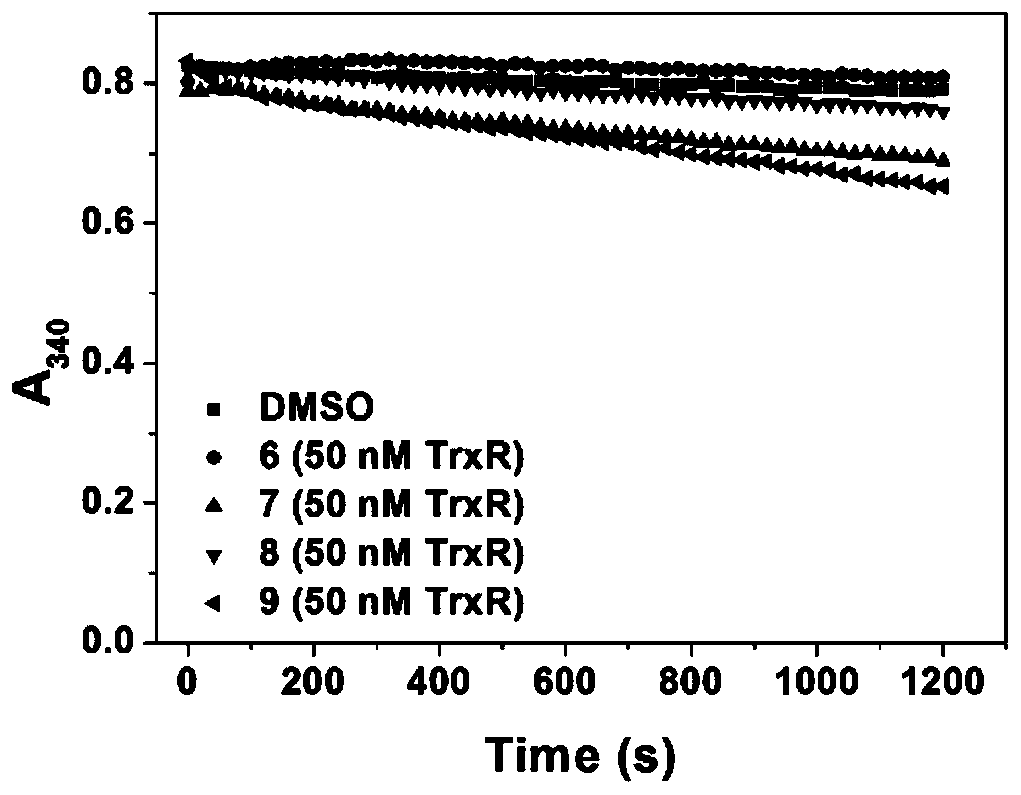
A kind of anticancer prodrug molecule and its preparation method and targeting compound
A compound and molecular technology, applied in the field of anti-cancer prodrug molecules and their preparation methods and targeted compounds, can solve the problems of short biological half-life, high toxicity, economic loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The embodiment of the present invention also provides a preparation method of an anticancer prodrug molecule, comprising:
[0042] The above-mentioned targeting compound is connected to the amino group or hydroxyl group of the anticancer active molecule to obtain the anticancer prodrug molecule.
[0043] Further, the active group is a hydroxyl group, and the preparation method includes:
[0044] React the -OH group of the targeting compound with triphosgene / phosgene to convert it into -OCOCl, and then combine -OCOCl with -NH in the anticancer active molecule 2 Or -OH group reaction.
[0045] The -OH group of the targeting compound reacts with triphosgene / phosgene and transforms into -OCOCl; when there is only -NH in the anticancer active molecule 2 When the group, -OCOCl and -NH 2 Group reaction; when there is only -OH group in the anticancer active molecule, -OCOCl reacts with -OH group; if there is -NH in the anticancer active molecule 2 or -OH group, the anti-can...
Embodiment 1
[0057] This embodiment provides a targeting compound whose structural formula is:
[0058]
[0059] In the formula, X is S or Se, R 1 , R 2 , R 3 At least one of them is an active group, and the active group includes any one of carboxyl, hydroxyl and amino.
[0060] Targeting compounds can be: One of them can also be other structural formula types; as long as the aforementioned general formula and limiting conditions are met.
[0061] Further, preferably R 1 and R 3 is hydrogen; R 2 selected from carboxyl, hydroxyl and amino groups. Then the general formula of the targeting compound is: One of.
Embodiment 2
[0063] The present embodiment provides an anticancer prodrug molecule (named S-Gem), its structural formula is:
[0064]
[0065] The reaction formula for preparing the anticancer prodrug molecule (S-Gem) is:
[0066]
[0067] Its preparation method is as follows:
[0068] S1. Dissolve the anticancer prodrug molecule gemcitabine Gem (1g, 3.8mmol) in 25mL of anhydrous DMF (dimethylformamide), add imidazole (0.78g, 11.5mmol) and TBDMSCl (tert-butyldimethylformamide) in sequence Chlorosilane) (1.5g, 9.6mmol); stirred at room temperature overnight, after the reaction was completed, the solvent was removed under reduced pressure, then extracted with ethyl acetate, the organic phase was washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, filtered, and spin-dried , separated by silica gel column chromatography to obtain TBSGem.
[0069] S2. Under argon protection, 1mmol cyclopentylamine was added to 25mL of anhydrous dichloromethane, then p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com