Enzymatic Chemiluminescent Substrates
An enzymatic chemiluminescence, substrate technology, applied in the field of immunodetection, can solve the problems of long-term stable preservation, limited application by storage conditions and use conditions, poor stability, etc., to improve thermal stability, real-time stability, duration The effect of long and fast entry into the plateau
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Enzymatic Luminescent Substrates
[0031] (1) Material: 5-(tetradecamido)fluorescein, 9-(4-chlorophenylthiobenzoyloxymethylene)-10-methyl-9,10-dihydroacridine-disodium Salt, MgCl 2 , ZnCl 2 , aryl acylhydrazone-9,10 acridine derivatives, cetyl trimethyl ammonium chloride are commercially available, chemically pure; Proclin-300, Tween20, Tris are products of sigma-aldrich in the United States. Among them, aryl acylhydrazone-9,10 acridine derivatives are synthesized from aryl acyl hydrazones and 9,10 acridine derivatives.
[0032] (2) Using Tris-Tween20 buffer as a solvent, different concentrations of enzymatic chemiluminescence substrates were prepared, as shown in Table 1.1-Table 1.4.
[0033] Table 1.1: Recipe one
[0034]
[0035] Table 1.2: Recipe II
[0036]
[0037]
[0038] Table 1.3: Recipe three
[0039]
[0040] Table 1.4: Recipe Four
[0041]
[0042]
[0043] In the enzymatic chemiluminescence substrate provided by the ...
Embodiment 2
[0045] Comparing the enzymatic chemiluminescence substrates of formula 1, formula 2, formula 3 and formula 4 with the commercially available Lumigen luminescent substrate, the AP signal intensity and linear relationship of each gradient concentration were tested. The details are shown in Table 2.1-Table 2.5:
[0046] Table 2.1
[0047]
[0048] Table 2.2
[0049]
[0050] Table 2.3
[0051]
[0052] Table 2.4
[0053]
[0054] Table 2.5
[0055]
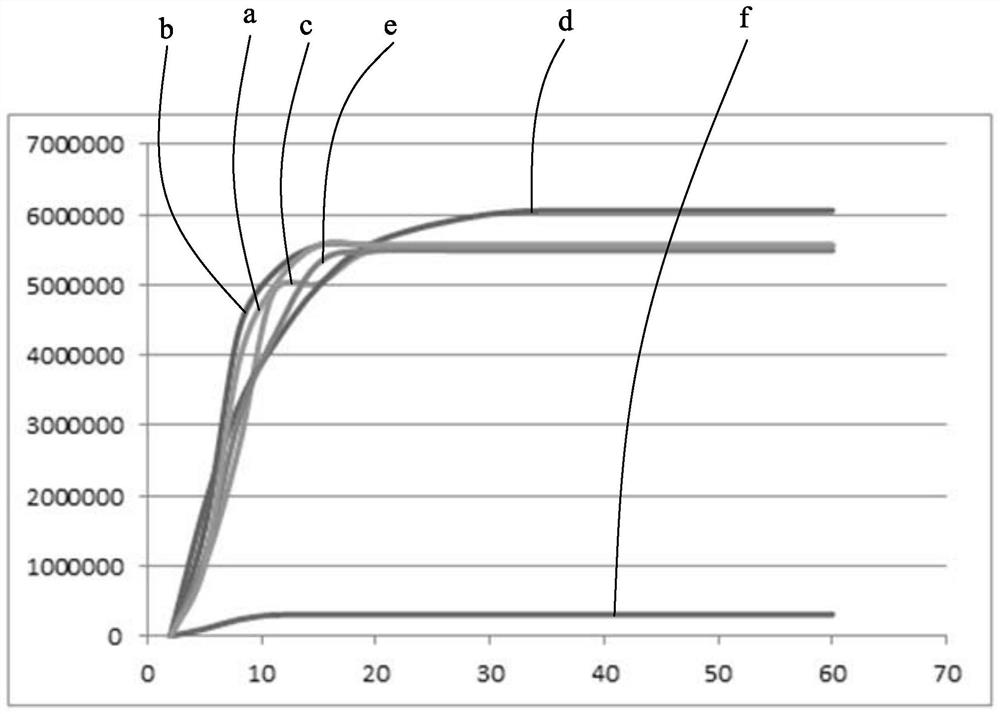
[0056] From the detection data results in Table 2.1-Table 2.5, it can be seen that the blank luminescence intensity of the enzymatic chemiluminescence substrate provided by the present invention is not higher than 300; under the condition of the same concentration of AP, the enzymatic chemiluminescence substrate provided by the present invention The signal intensity is higher than that of the commercially available Lumigen luminescent substrate.
Embodiment 3
[0058] The linearity of the enzymatic chemiluminescence substrates of formula 1, formula 2, formula 3 and formula 4 was compared with that of the commercially available Lumigen luminescent substrates for the determination of carcinoembryonic antigen (CEA) quantitative detection kits.
[0059] The double antibody sandwich method was used to detect the content of CEA in human serum. The experimental steps were as follows:
[0060] Step S1: Add 30ul of human serum sample or CEA calibrator, 100ul of diluted enzyme-labeled antibody, and 30ul of antibody-conjugated magnetic beads into the chemiluminescence tube, respectively, and react at 37°C for 15min after mixing;
[0061] Step S2: add 300ul of cleaning solution to clean 3 times;
[0062]Step S3: adding 100ul of the enzymatic chemiluminescence substrates of formula 1, formula 2, formula 3, formula 4 and commercially available chemiluminescence substrates of Lumigen into different chemiluminescence tubes respectively;
[0063] St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com