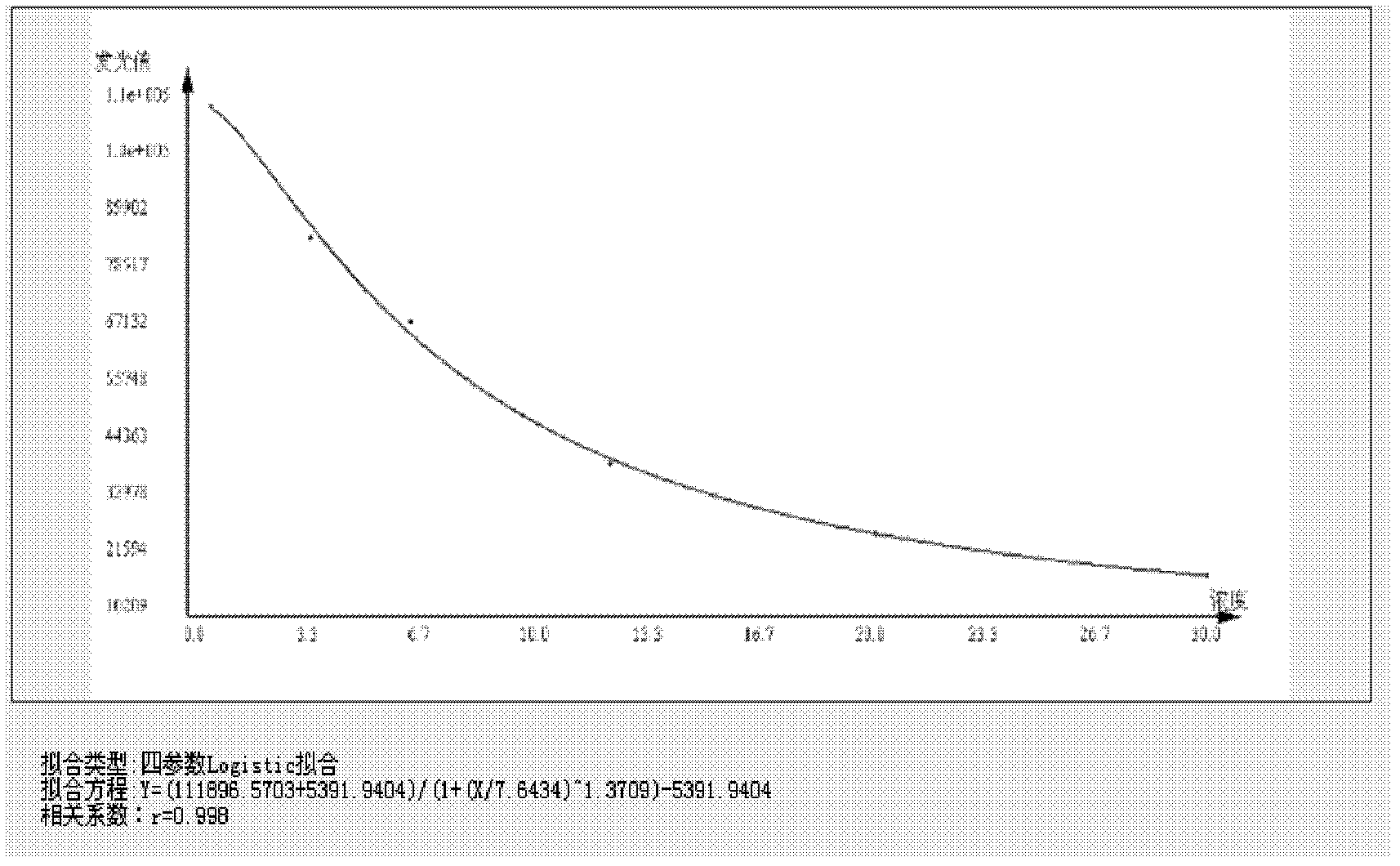Chemiluminescence ELISA kit for detecting concentration of drug tacrolimus in human whole blood and Chemiluminescence bottom indicator
A chemiluminescence enzyme and chemiluminescence technology, which is applied in the field of medical detection, can solve the problem that the luminescence performance cannot meet the sensitivity and stability of the detection requirements, affect the sensitivity and stability of the detection kit, and affect the monitoring results of the patient's blood drug concentration, etc. problem, to achieve the effect of shortening the detection time, high accuracy and long duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Preparation and use of a kit in which tacrolimus monoclonal antibody is the coating source and the marker is an enzyme-labeled hapten
[0027]1. The detection principle of the kit using tacrolimus monoclonal antibody as the coating source and the enzyme-labeled tacrolimus hapten as the marker is as follows:
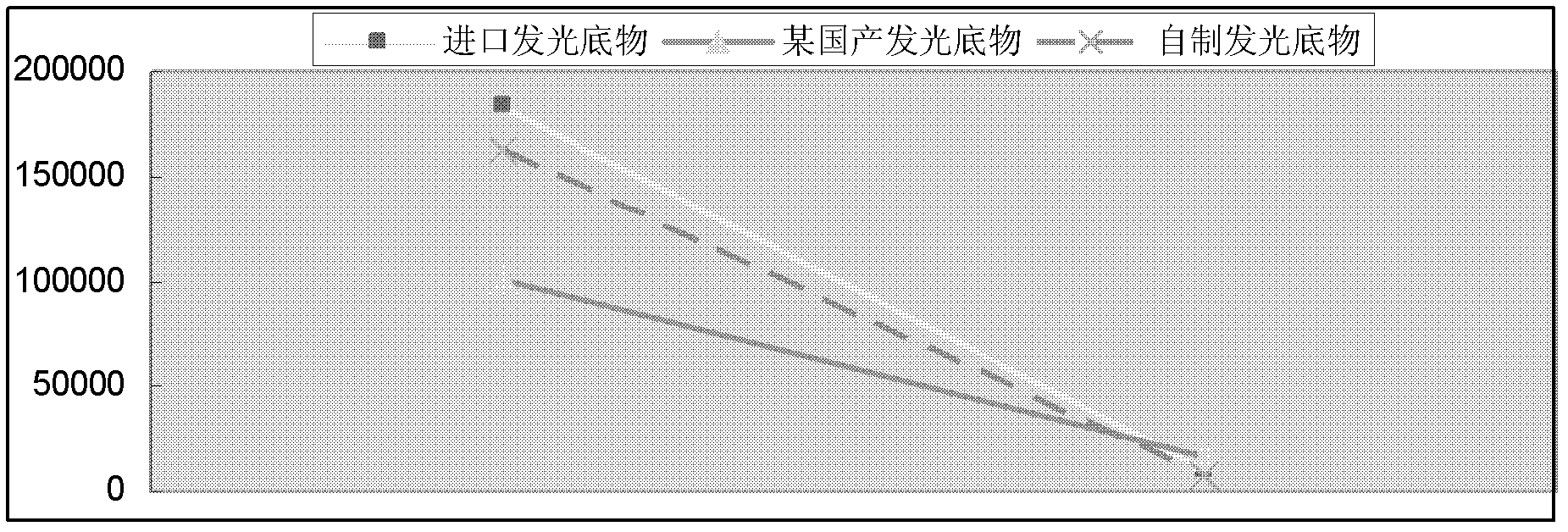
[0028] The microwell strip is pre-coated with tacrolimus monoclonal antibody, and the tacrolimus in the sample and the enzyme-labeled tacrolimus compete with the pre-coated anti-tacrolimus antibody on the microwell strip , so that the absorbance value of the sample is negatively correlated with the content of tacrolimus contained in the sample, and the amount of tacrolimus in the sample can be obtained by comparing with the standard curve.
[0029] 2. Kit composition:
[0030] Kit No. I:
[0031] 1. Chemiluminescent microtiter plates coated with a coating source: the coating source is a mouse monoclonal antibody against tacrolimus, and the coating conc...
Embodiment 2
[0054] Weigh 1 mg of the above hapten and dissolve it in 2 ml of N, N-dimethylformamide, stir at room temperature for 30 min, add dropwise to 10 ml (5 mg) of horseradish peroxidase solution, stir overnight at room temperature, and use Sephadex G -25 for chromatographic purification. Example 2. Application of a kit using tacrolimus monoclonal antibody as a coating source and an enzyme-labeled hapten as a marker
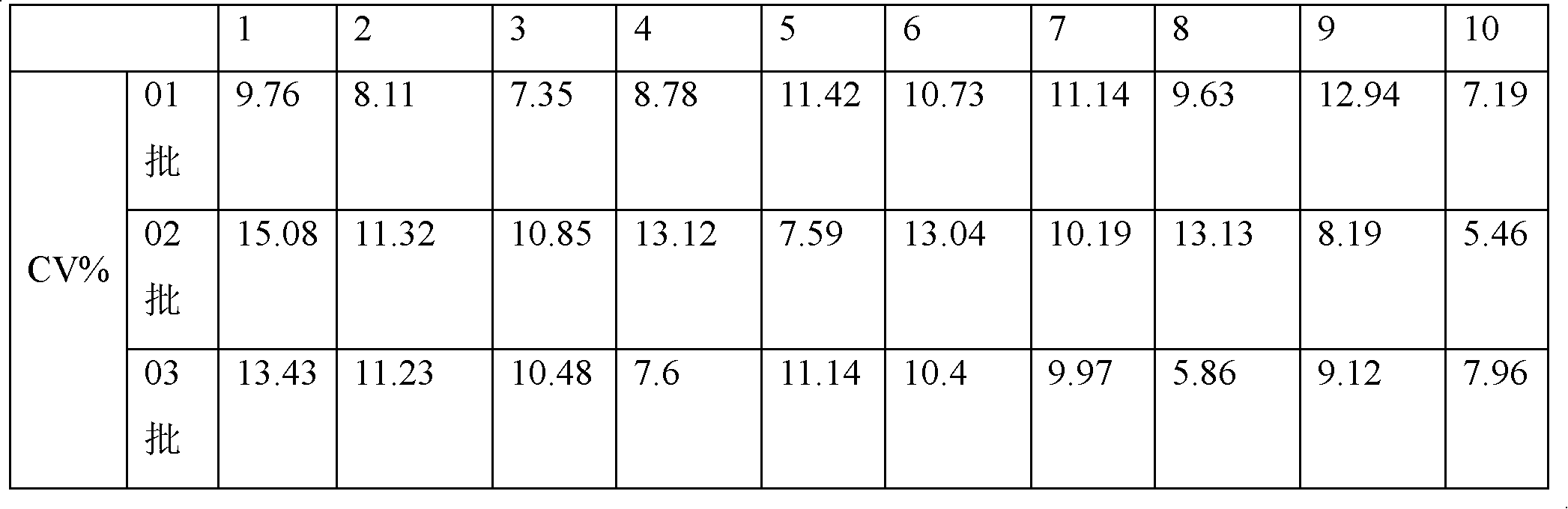
[0055] The kit of the invention is used for quantitatively detecting the drug concentration of tacrolimus in human whole blood.
[0056] 1. Pretreatment of human whole blood samples
[0057] Shake the human whole blood tacrolimus standard and quality control solution and samples to be tested stored in the refrigerator at 2-8°C, fully shake and mix on the shaker, and immediately pipette 150 μl of each sample into the corresponding centrifuge tube accurately, After adding an equal volume of sample pretreatment solution, cap the tube immediately, rotate at high speed wi...
Embodiment 3
[0091] Example 3. The original coating is a conjugate of tacrolimus and carrier protein, and the enzyme-labeled secondary antibody is an enzyme-labeled kit
[0092] The working principle of this kit is:
[0093] When the coating on the microwell strip of the chemiluminescent microtiter plate is originally a conjugated tacrolimus hapten and carrier protein, after adding the standard solution or sample solution to the microwell of the chemiluminescent microtiter plate, add the antibody The specific antibody of tacrolimus, the tacrolimus in the sample competes with the tacrolimus-coupled antigen on the chemiluminescence microtiter plate, and then the enzyme-labeled anti-antibody is added for amplification, and the substrate is used for color development In this way, the absorbance value of the sample is negatively correlated with the content of tacrolimus, and the content of tacrolimus in the sample can be obtained according to the standard curve obtained from the standard.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com