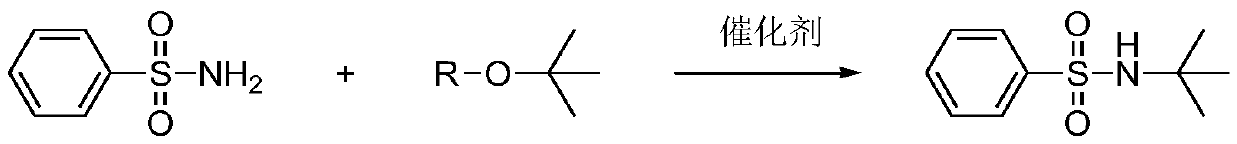A kind of synthetic method of n-tert-butylbenzenesulfonamide
A technology of tert-butylbenzenesulfonamide and synthesis method, which is applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve the problems of poor reactivity of tert-butyl ether, expensive catalyst, difficult-to-react raw materials, etc., and achieves environmental friendliness and industrial waste. Reduced, active and inexpensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 31.81mmol of benzenesulfonamide, 47.72mmol of tert-butanol, 0.9543mmol of hafnium tetrachloride into a four-necked flask with a thermometer and a reflux condenser, then add 30mL of N-methylpyrrolidone as a solvent, heat to 150°C, and perform HPLC Monitor the reaction (chromatographic conditions: the mobile phase ratio is methanol-water 70:30, the detection wavelength is 254nm), and the disappearance of the raw material benzenesulfonamide is the end of the reaction. The system was cooled to room temperature, the insoluble matter was filtered off, and the filtrate was precipitated under reduced pressure to obtain a yellow liquid, which was the product, with a yield of 97.5%, a purity of 98.8% (HPLC), and MS m / z: 213.08 (M+1,100).
Embodiment 2
[0020] Add 31.81mmol of benzenesulfonamide, 51.36mmol of tert-butyl propionate, and 0.3181mmol of hafnium tetrachloride into a four-neck flask with a thermometer and a reflux condenser, then add 30mL of dimethyl sulfoxide as a solvent, and heat to 139°C. The reaction was monitored by HPLC (chromatographic conditions: the mobile phase ratio was methanol-water 70:30, the detection wavelength was 254nm), and the disappearance of the raw material benzenesulfonamide was the end of the reaction. The system was cooled to room temperature, the insoluble matter was filtered off, and the filtrate was precipitated under reduced pressure to obtain a yellow liquid, which was the product, with a yield of 96.0%, a purity of 98.8% (HPLC), and MS m / z: 213.08 (M+1,100).
Embodiment 3
[0022] Add 31.81mmol of benzenesulfonamide, 43.18mmol of tert-butyl propionate, and 3.181mmol of hafnium tetrachloride into a four-necked flask with a thermometer and a reflux condenser, then add 30mL of xylene as a solvent, heat to 155°C, and monitor by HPLC Reaction (chromatographic conditions: the mobile phase ratio is methanol-water 70:30, the detection wavelength is 254nm), and the disappearance of the raw material benzenesulfonamide is the end of the reaction. The system was cooled to room temperature, the insoluble matter was filtered off, and the filtrate was precipitated under reduced pressure to obtain a yellow liquid, namely the product, with a yield of 95.5%, a purity of 98% (HPLC), and MS m / z: 213.08 (M+1,100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com