Antipyretic and analgesic ibuprofen sustained-release capsule composition and preparation method thereof
A technology of antipyretic, analgesic and slow-release capsules, which is applied in the field of medicine, can solve the problems of large dosage of ibuprofen, and achieve the effects of low toxic and side effects, good tolerance and high drug dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
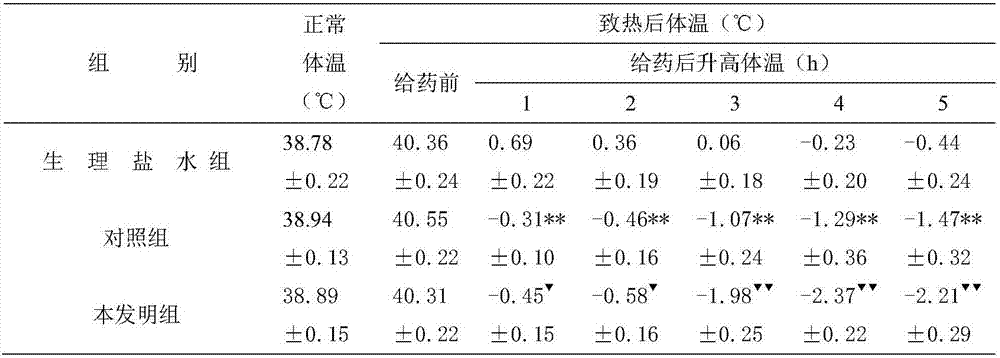
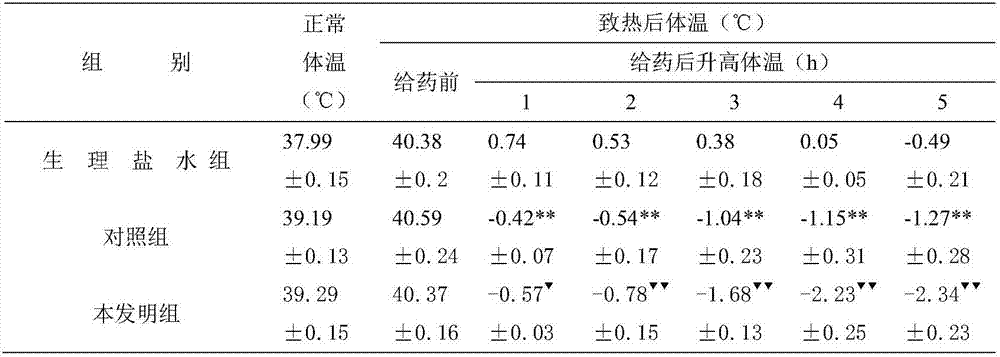
[0017] Embodiment 1: get ibuprofen 200g, tulobuterol hydrochloride 100g, blank ball core 60g, carboxymethyl cellulose 30g, carboxymethyl starch sodium 20g and magnesium stearate 5g, preparation method comprises the steps: adopt centrifugal To prepare granules by granulation, pour the blank core into the centrifugal granulator, turn on the centrifugal turntable, set the speed to 100-120rpm, turn on the peristaltic pump, adjust the atomization pressure, spray ethanol, and sprinkle ibuprofen and hydrochloric acid at the same time Tulobuterol, carboxymethyl cellulose, and sodium carboxymethyl starch are used for drug application. After the drug application is completed, the pellets are dried, and the pellets between 16 mesh and 26 mesh are taken, and magnesium stearate is added. Carry out capsule filling, then aluminum-plastic packaging gets final product.
Embodiment 2
[0018] Embodiment 2: get ibuprofen 200g, tulobuterol hydrochloride 200g, blank ball core 60g, polyvinylpyrrolidone 30g, croscarmellose sodium 20g and magnesium stearate 5g, preparation method comprises the steps: using To prepare granules by centrifugal granulation, pour the blank core into the centrifugal granulator, turn on the centrifugal turntable, set the speed at 100-120rpm, turn on the peristaltic pump, adjust the atomization pressure, spray ethanol, and sprinkle ibuprofen, Tulobuterol hydrochloride, polyvinylpyrrolidone, and croscarmellose sodium are used for drug application. After the drug application is completed, the pellets are dried, and the pellets between 16 mesh and 26 mesh are taken, and stearic acid is added. Magnesium, for capsule filling and then aluminum-plastic packaging.
Embodiment 3
[0019] Embodiment 3: get ibuprofen 200g, tulobuterol hydrochloride 150g, blank ball core 60g, hydroxypropyl methylcellulose 30g, crospovidone 20g and magnesium stearate 5g, the preparation method comprises the following steps: Prepare granules by centrifugal granulation method, pour the blank core into the centrifugal granulator, turn on the centrifugal turntable, set the speed at 100-120rpm, turn on the peristaltic pump, adjust the atomization pressure, spray ethanol, and sprinkle ibuprofen at the same time , tulobuterol hydrochloride, hydroxypropyl methylcellulose, and crospovidone are used for drug application. After the drug application is completed, the pellets are dried, and the pellets between 16 mesh and 26 mesh are taken, and hard Magnesium fatty acid, capsule filling, and then aluminum-plastic packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com