Method for inactivating viruses in protein solution by adopting ultraviolet irradiation
A technology of protein solution and ultraviolet rays, which is applied in the direction of irradiation, disinfection, sanitary equipment for toilets, etc., can solve the problem of destruction of effective protein components and achieve good inactivation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Flow type protein solution ultraviolet inactivation instrument
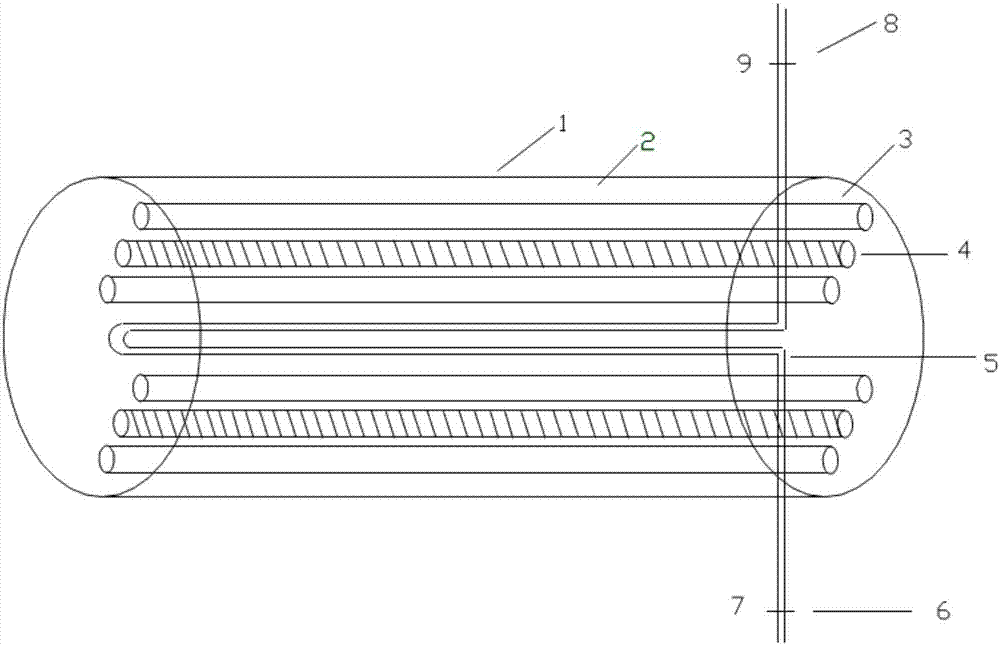
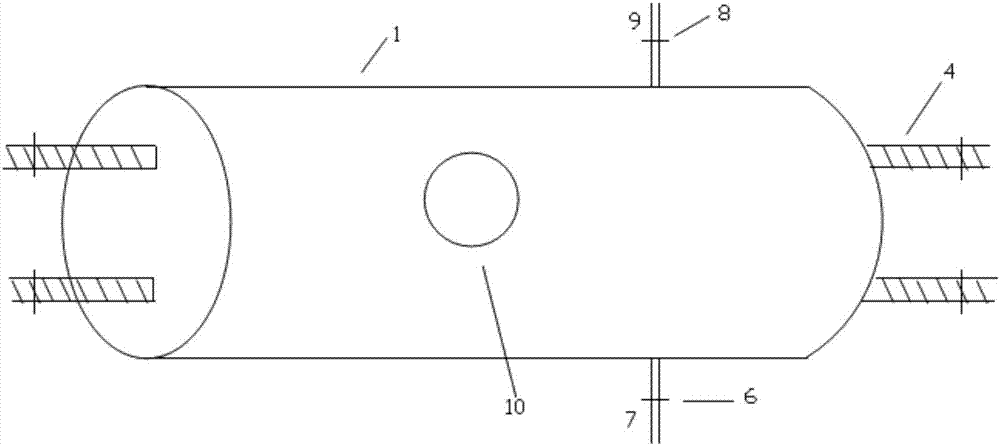
[0046] like Figure 1 ~ Figure 3 As shown, the flow type protein solution ultraviolet inactivation instrument used in the present invention comprises a cylinder 1, the cylinder is a dark box, the inside of the cylinder is coated with a reflective coating 2, and the side of the cylinder is provided with an openable movable panel 10 , and the movable panel is provided with a visual window, which is convenient for observing the situation in the cylinder, and through this window, the probe of the ultraviolet intensity meter can be inserted into the cylinder to measure the intensity of ultraviolet rays, or the thermometer can be inserted into the cylinder to measure the temperature.
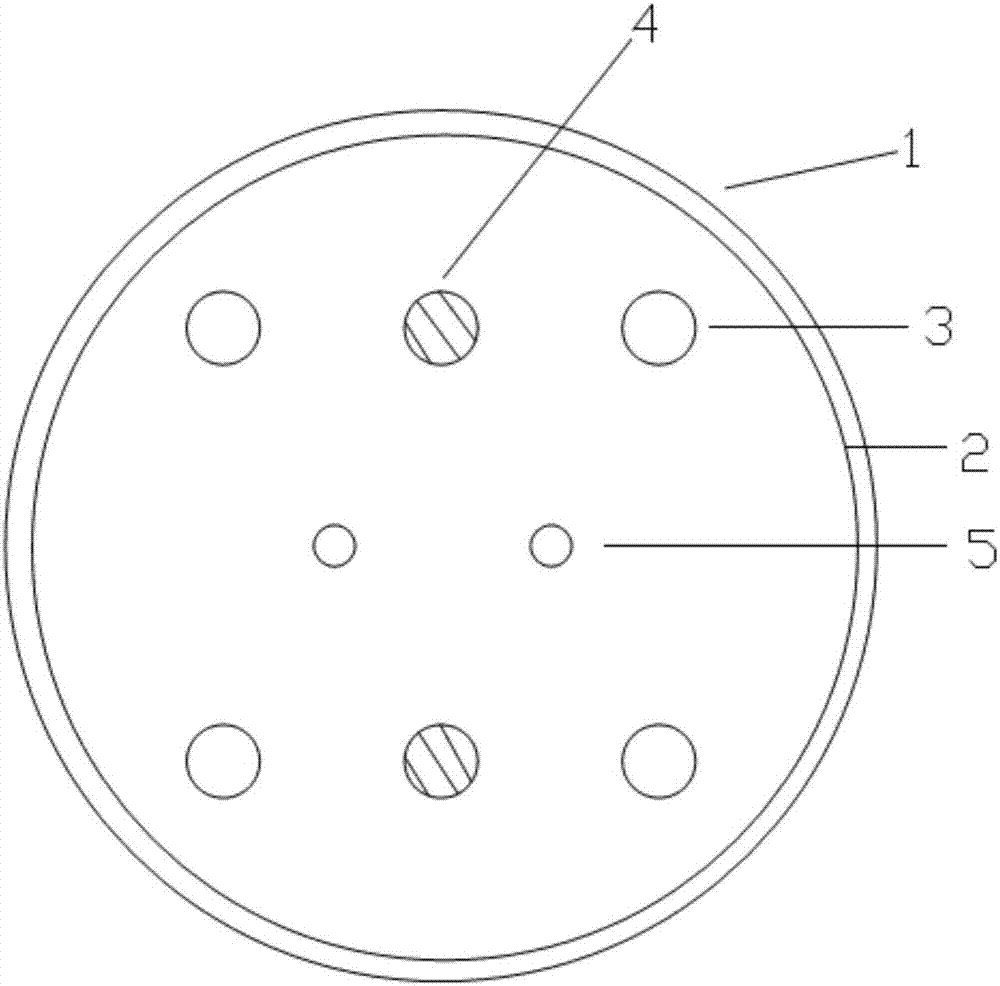
[0047] Two sets of UV lamp groups 3 arranged symmetrically up and down are fixed in the cylinder body 1. The purpose of symmetrical placement is to ensure that the protein solution can be irradiated with ultraviolet ray...
Embodiment 2
[0053] Example 2: Flow type protein solution ultraviolet inactivation instrument
[0054] like Figure 4~5 As shown, in the flow-type protein solution ultraviolet inactivation instrument of this embodiment, the protein solution flow tube 5 is in a spiral shape, and what is selected is a quartz glass tube. The input end of the protein solution flow pipe 5 passes through the lower end of the side panel of the cylinder body 1 and is arranged outside the cylinder body, and the output end of the protein solution flow pipe 5 passes through the upper end of the side panel of the cylinder body 1 and is arranged outside the cylinder body, and the protein solution flows The input end and output end of the tube 5 are respectively located at the two ends of the barrel. The structure of other parts of the flow-type protein solution ultraviolet inactivation instrument of this embodiment is the same as that of Embodiment 1.
[0055] In other embodiments, the protein solution flow pipe can ...
Embodiment 3
[0056] Example 3: Inactivation verification of porcine parvovirus (PPV) in fibrinogen
[0057] The cultivation of porcine parvovirus (PPV): add the porcine parvovirus (PPV) liquid into a monolayer or overgrow 90% porcine testicular cells (ST cells, Image 6 ), after 2 hours of adsorption, discard the virus liquid, add DMEM medium containing 2% fetal bovine serum (FBS) to culture the virus, and collect the virus liquid when 3 / 4 cells have lesions after about 72 hours. The culture medium containing the virus and host cells was placed at -20°C and repeatedly frozen and thawed 3 times to break up the host cells and release the virus. Then, centrifuge at 4° C. (3000 rpm, 10 min) to remove cell debris, and the supernatant is the desired virus suspension.
[0058] Determination of virus titer: 10-fold serial dilution of the virus stock solution was performed with DMEM culture solution containing 2% FBS. Take a 96-well cell culture plate covered with a monolayer of host cells, pour ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Strength | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com