Copper (ii) complex constructed by ibuprofen and quinoline-8-formaldehyde Schiff base and its synthesis method and application
A synthesis method and complex technology, which is applied in the field of medicine, can solve problems such as no synthesis method and achieve good proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take the ligand L2 and [Cu(L1) of the amount (1.0mmol) of equal substance 2] placed in a 50ml round bottom flask, then add 20ml of methanol, stir at room temperature for 24h, filter, collect the filtrate in a 50ml beaker, seal with plastic wrap, then pierce small holes on the plastic wrap, volatilize at room temperature, and obtain a block after one week Dark green crystals, yield 80%.
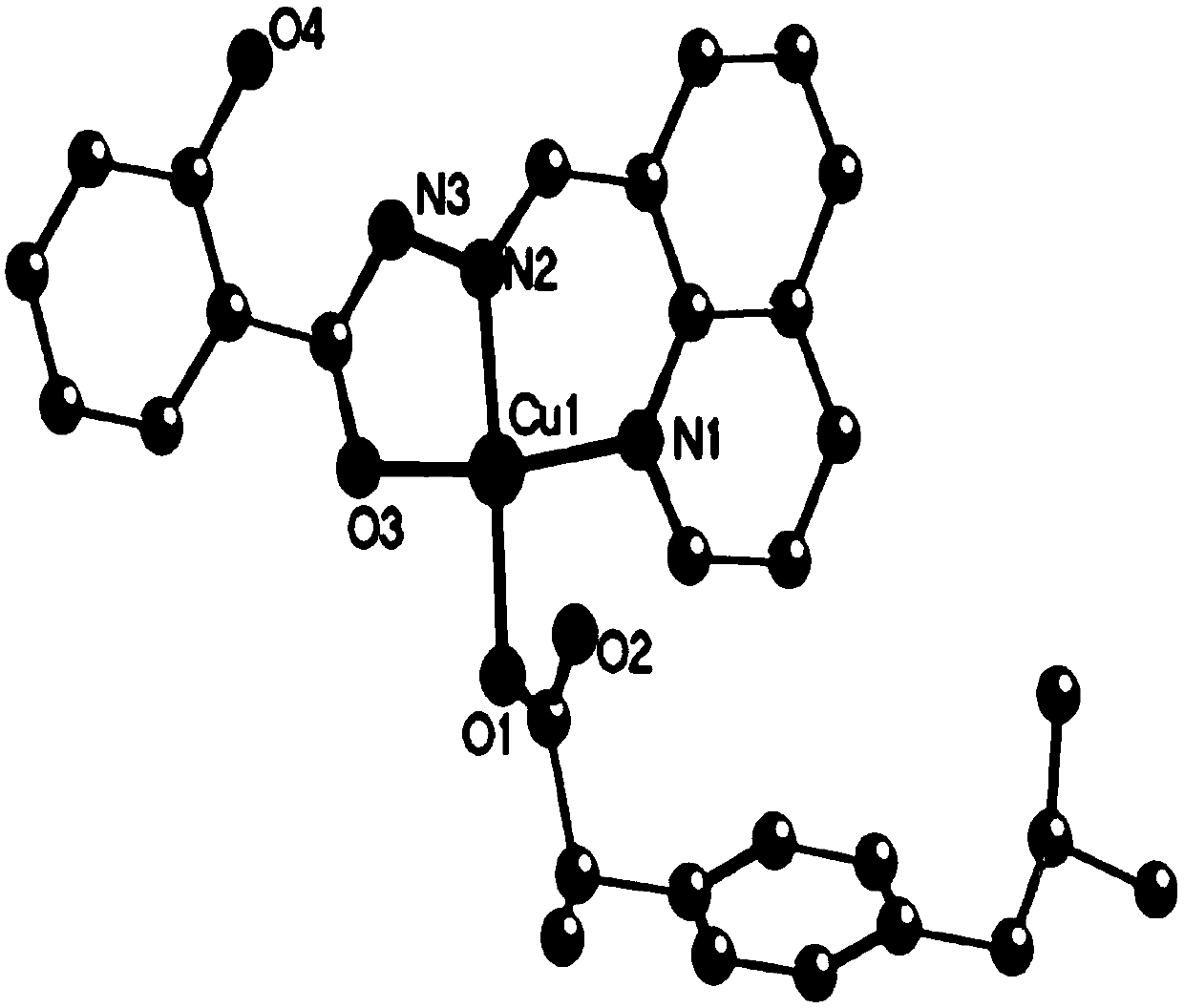
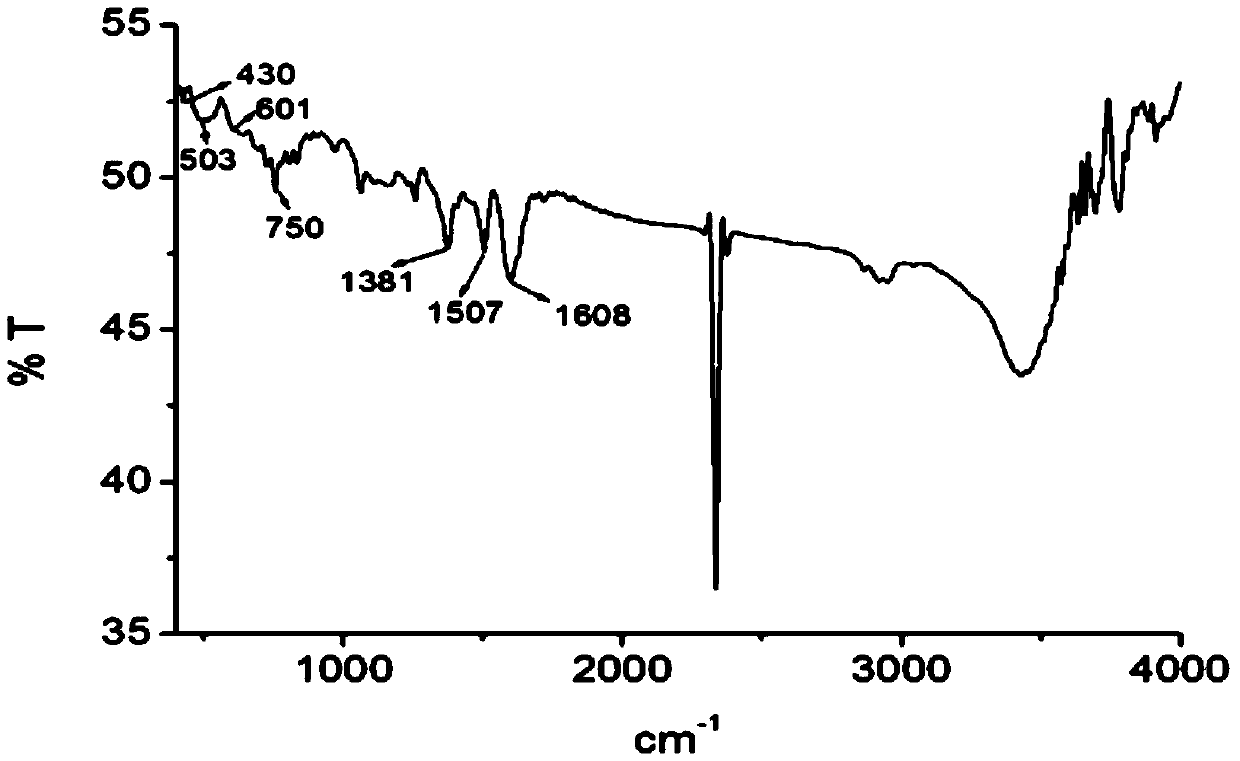
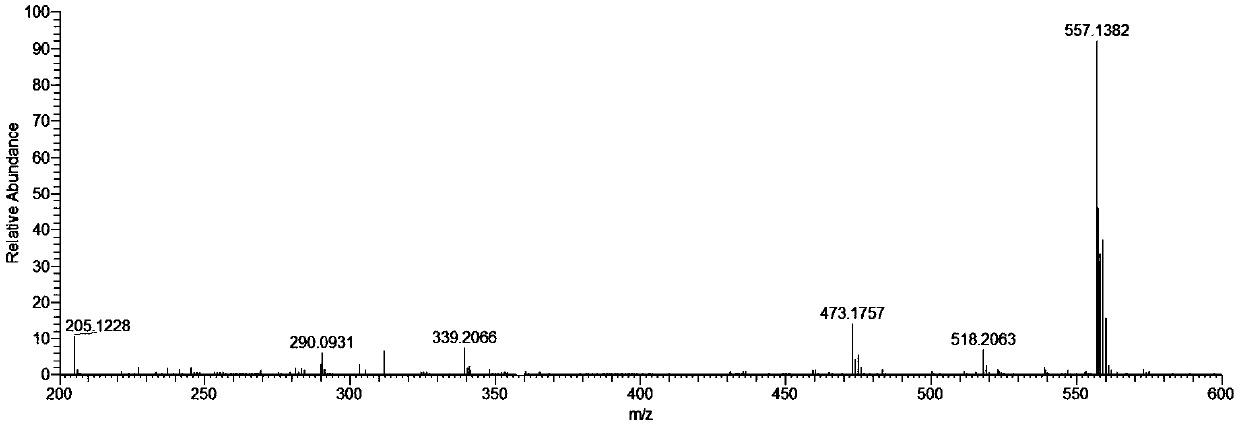
[0040] The obtained crystal of this embodiment carries out X-ray single crystal diffraction, infrared spectrum and high-resolution mass spectrometry analysis, and its collection of illustrative plates is respectively as follows figure 1 , figure 2 with image 3 As shown, therefore, it can be determined that the blocky dark green crystals obtained are [Cu(L1)(L2)] of the present invention, wherein L1 is ibuprofen with a negative charge of one unit; L2 is quinoline- 8-Formaldehyde salicylhydrazone Schiff base, with a negative charge of one unit.
Embodiment 2
[0046] Example 1 was repeated except that the solvent was changed to ethanol. After ten days, massive dark green crystals were obtained with a yield of 78%.
[0047] Carry out X-ray single crystal diffraction, infrared spectrum and high-resolution mass spectrometry analysis to the crystal obtained in this embodiment, can confirm that the block dark green crystal of gained is [Cu(L1)(L2)] described in the present invention, wherein, L1 is ibuprofen with one unit of negative charge; L2 is quinoline-8-formaldehyde salicylhydrazone Schiff base with one unit of negative charge.
Embodiment 3
[0049] Repeat Example 1, the difference is that the solvent is changed to a mixed solvent composed of methanol and methylene chloride (volume ratio is 4:1), and the total amount of solvent used remains unchanged. One week later, massive dark green crystals were obtained with a yield of 75%.
[0050] Carry out X-ray single crystal diffraction, infrared spectrum and high-resolution mass spectrometry analysis to the crystal obtained in this embodiment, can confirm that the block dark green crystal of gained is [Cu(L1)(L2)] described in the present invention, wherein, L1 is ibuprofen with one unit of negative charge; L2 is quinoline-8-formaldehyde salicylhydrazone Schiff base with one unit of negative charge.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com