Fluorescent probe containing active molecules of flavonoids and its preparation method and application
A technology of drug active molecules and fluorescent probes, which is applied in the field of fluorescent probes containing flavonoid drug active molecules and its preparation and application, can solve the problems of complex components, restrictions on the clinical application of natural product drugs, and the inability to determine the molecular mechanism of action. Biological function pathways and other issues to achieve good proliferation inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
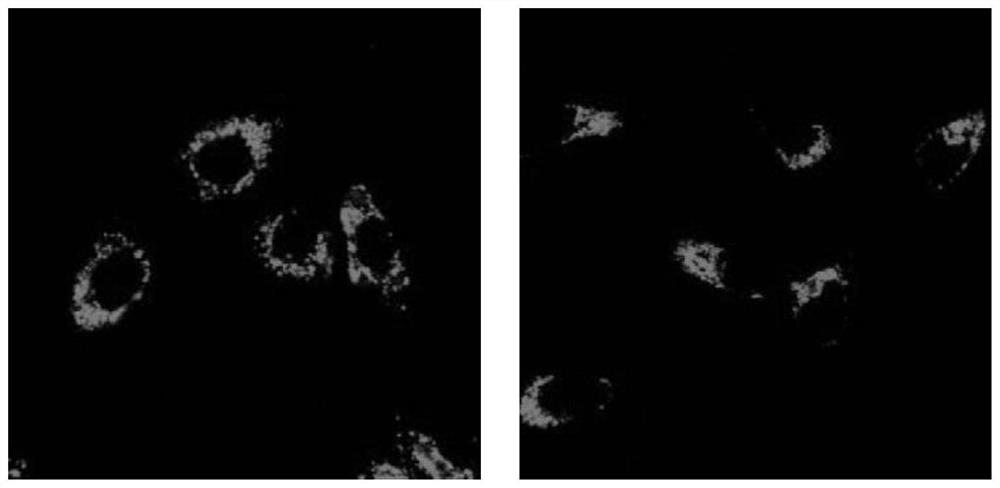
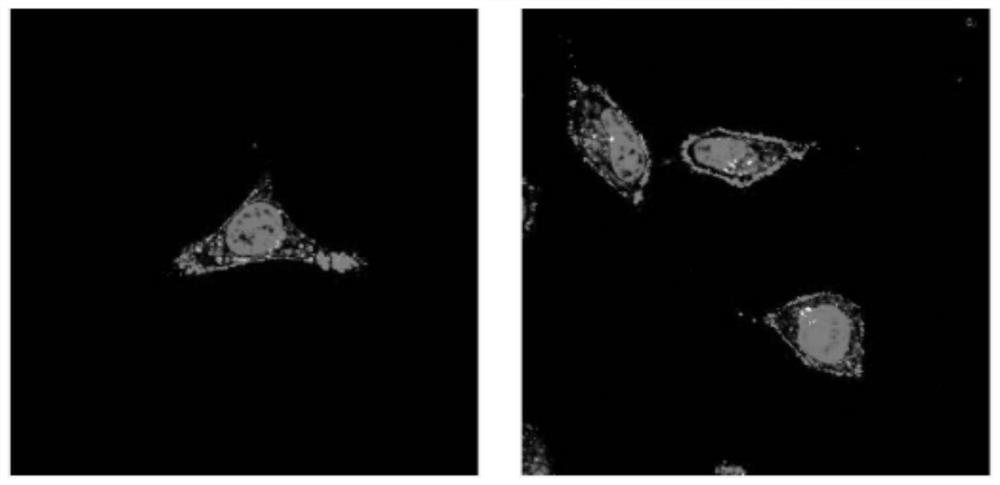
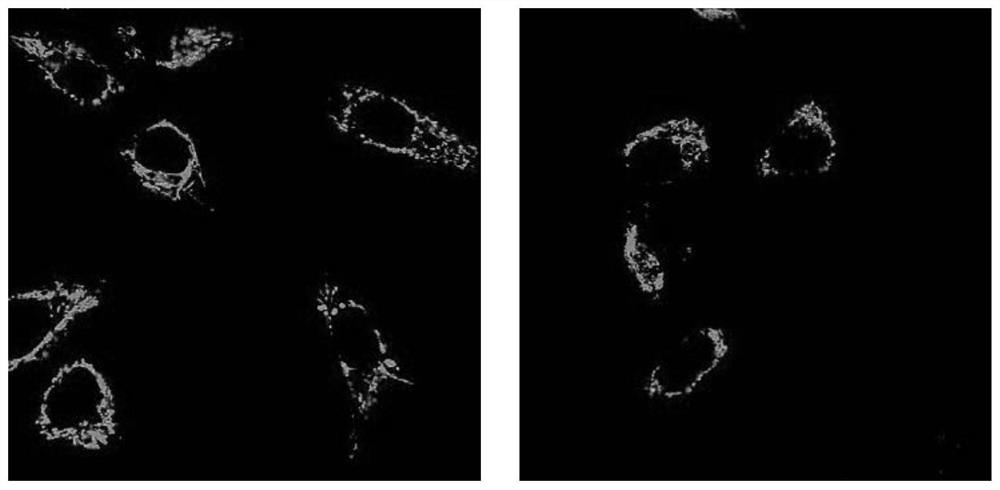
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing the above-mentioned fluorescent probe containing flavonoid drug active molecules, wherein the method comprises the following steps,
[0042] 1) make the compound of structure shown in formula (2) and Carrying out an amide condensation reaction to obtain an intermediate compound represented by the general formula L-Y, wherein n is an integer of 1-6;
[0043] 2) Mannich reaction is carried out between the intermediate compound represented by the general formula L-Y and the compound represented by the formula (1), to obtain a fluorescent probe containing active molecules of flavonoids represented by the general formula X-L-Y.
[0044] According to the present invention, the compound of the structure shown in formula (2) can be commercially available, and can also be synthesized after optimization by conventional methods in the art. When the synthesis is obtained, for example, references (1) Anzalone, A.V.; Wang, ...
preparation example 1
[0079] This preparation example is used to illustrate the preparation of the compound with the structure shown in formula (2).
[0080]
[0081](1) Add 3.23g (20mmol) of 7-hydroxy-2-quinolone (compound 1) into 90mL tetrahydrofuran, slowly add 1.22g (32mmol) lithium aluminum hydride with stirring in an ice bath, heat to reflux, and react overnight. After the reaction was stopped, it was cooled, and 35.0 ml of saturated ammonium chloride solution was added immediately to quench the reaction, extracted with ethyl acetate, and the organic phase was washed with saturated sodium chloride solution, and then concentrated to obtain 2.83 g of a yellow solid with a yield of 95%. ESI-MS(m / z):[M+H] + calcd.for C 9 h 11 NO, 150.09; found, 150.25.
[0082] (2) Add 2.50 g (16.8 mmol) of 7-hydroxy-1,2,3,4-tetrahydroquinoline (compound 2) to 65 mL of acetic acid, and slowly add 2.53 g (67.2 mmol) of sodium borohydride while stirring at room temperature. When about half of the raw materia...
preparation example 2
[0089] This preparation example is used to illustrate the preparation of the compound with the structure shown in formula (6).
[0090]
[0091] (1) Mix 400mg (0.78mmol) of the compound of the structure shown in the formula (2-1) and the mixture of the compound of the structure shown in the formula (2-2) (the mixing molar ratio is 1:1), 179.5mg (0.94mmol) 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI), 126.5 mg (0.94 mmol) 1-hydroxybenzotriazole (HOBT) and 325.5 μL triethylamine were added Into 100mL of dry dichloromethane, stirred at 50°C for 1-2h. Add 285 μL of 1,8-diamino-3,6-dioxahexane and continue the reaction for 24 hours. Add water to terminate the reaction, extract and concentrate to obtain a mixture of the compound represented by formula (6-1) and the compound represented by formula (6-2), which is a dark red solid (0.20 g, yield 40.3%). A part was taken out from the above mixture and separated to obtain the compound with the structure shown in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com