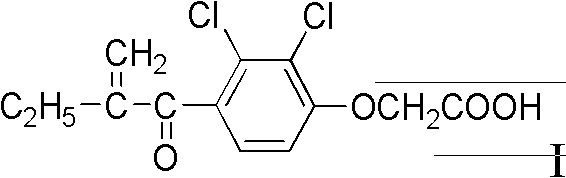
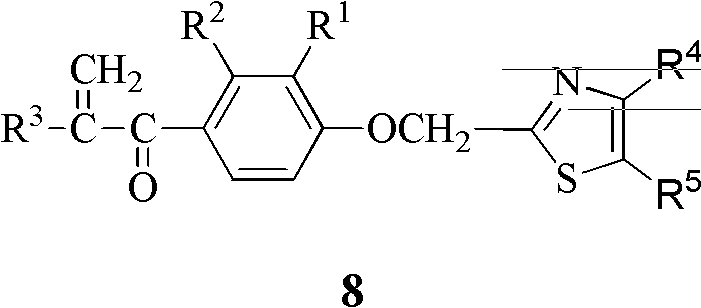
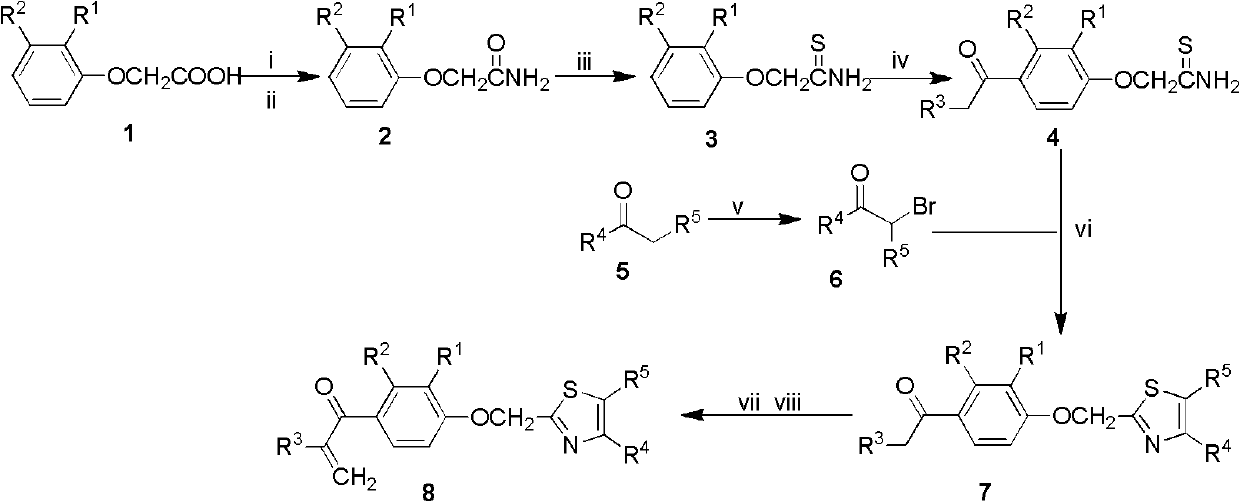
Alpha, beta-unsaturated ketone compound containing thiazole heterocycle, its preparation method and application
A ketone compound, unsaturated technology, applied in the field of thiazole-containing heterocycle α, can solve the problem of lack of isozyme specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the thiazole heterocyclic α, β-unsaturated ketone compound containing general formula 8 of the present invention, with R 1 is hydrogen or methyl, R 2 is methyl or chlorine, R 3 is methyl or ethyl, R 4 is phenyl, p-nitrophenyl, p-trifluoromethylphenyl or 2-naphthyl, R 5 For hydrogen as an example, the steps are as follows:
[0041] (1) Mix 2-(substituted phenoxy)acetic acid 1 and thionyl chloride at a molar ratio of 1:10, heat to 80°C, react for 4 hours, distill off thionyl chloride under reduced pressure, and dilute with anhydrous toluene , add the obtained toluene solution to ammonia water under ice-bath conditions, continue to stir for 1.5h, filter, and recrystallize the filter cake with ethanol-water to obtain intermediate compound 2: 2-(substituted phenoxy)acetamide 2 ;
[0042] (2) Add the above-mentioned intermediate compound 2 and phosphorus pentasulfide into tetrahydrofuran in a molar ratio of 1:2, use 250 ml of tetrahydrofuran per...
Embodiment 1
[0054] Embodiment 1. Preparation of Compounds 8a~8o
[0055] (1) Preparation of 2-(substituted phenoxy)acetamide 2
[0056] Add 2-(substituted phenoxy)acetic acid 1 (20mmol) and thionyl chloride 15mL (200mmol) into a 50mL round-bottomed flask, stir and mix, slowly heat the oil bath to 80°C, reflux for 4h, evaporate under reduced pressure Remove the residual thionyl chloride in the reaction solution, add 10 mL of dry toluene to dilute and directly use it in the next reaction.
[0057] Take a 100mL three-necked flask (with a drying tube) and place it in an ice-water bath, add 10mL of ammonia water, and slowly add the reaction solution treated in the previous step into the ammonia water. Remove to filtrate. The filter cake was transferred to hot acetone, fully dissolved and filtered, the filter residue was washed with hot acetone, the lotion and filtrate were combined, and the solvent was evaporated under reduced pressure. The obtained white solid was recrystallized with ethano...
Embodiment 2
[0119] Embodiment 2: Determination of compound's inhibitory activity on GST Pi and growth inhibitory activity on HL-60 cells
[0120] Compounds 8a-8o prepared in Example 1 were tested for their inhibitory activity by the following method, and the results are listed in Table 1.
[0121] For the assay method of GST Pi inhibitory activity, according to the prior art, refer to Example 5 on page 11 of CN1706789 "α, β-unsaturated ketone compound and its preparation method and its inhibition of GSTπ activity" (200510043573.3).
[0122] For the assay method of HL-60 cell growth inhibitory activity, according to the prior art, refer to page 3 of CN101108832 "five-membered heterocyclic compounds, preparation method and application" (200710015199.5).
[0123] Table 1. Determination data of compounds inhibiting GST Pi activity and inhibiting HL-60 cell growth activity
[0124]
[0125]
[0126]
[0127] The experimental results show that the 15 kinds of α, β-unsaturated ketone c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com